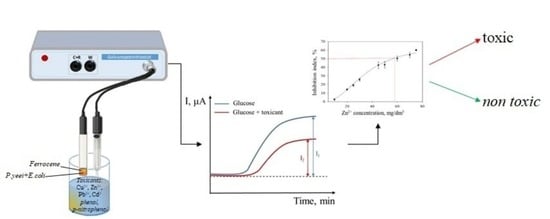
Mediator Microbial Biosensor Analyzers for Rapid Determination of Surface Water Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Microorganisms
2.3. Cultivation of Microbial Cells
2.4. Formation of Working Electrode
2.5. Registration of Current–Voltage Characteristics
2.6. Biosensor Measurements
2.7. Reference Toxicity Tests: Assessment of Chlorella Growth Inhibition
2.8. Biosensor Determination of Water Sample Toxicity
3. Results
3.1. Choice of Analytical Signal Registration Method
3.2. Choice of Mediator and Microorganisms for Toxicity Index Assessment
| Mediator | Rate Constants of the Interaction of Mediators with Investigated Microorganisms, dm3/g × s | Rate Constant of the Heterogeneous Transfer to the Electrode, cm·s−1 [27] | |||
|---|---|---|---|---|---|
| Ps. Veronii | E. coli | S. cerevisiae | P. yeei [27] | ||
| Methylene blue | 2.0 ± 0.5 | 0.20 ± 0.04 | 0.06 ± 0.01 | 0.021 ± 0.001 | 0.025 ± 0.009 |
| Thionine | 0.05 ± 0.01 | 0.010 ± 0.002 | 0.5 ± 0.1 | 0.013 ± 0.004 | 0.022 ± 0.005 |
| Neutral red | 0.020 ± 0.003 | 0.08 ± 0.01 | 0.6 ± 0.1 | 0.013 ± 0.003 | 0.017 ± 0.005 |
| Potassium hexacyanoferrate(III) | 0.20 ± 0.03 | 0.010 ± 0.002 | 0.002 ± 0.001 | 0.019 ± 0.003 | 0.0067 ± 0.0009 |
| 2,6-Dichloro-phenolindophenol | 0.08 ± 0.02 | 0.010 ± 0.002 | 0.3 ± 0.1 | 0.013 ± 0.002 | 0.069 ± 0.004 |
| Ferrocene | 0.00020 ± 0.00002 | 0.030 ± 0.006 | 0.3 ± 0.1 | 0.023 ± 0.001 | 0.4 ± 0.1 |
| 1,1′-Dimethyl-ferrocene | 0.00020 ± 0.00004 | 0.00030 ± 0.00002 | 0.00030 ± 0.00008 | 0.0038 ± 0.0009 | 0.07 ± 0.01 |
| Ferrocene carboxaldehyde | 0.10 ± 0.05 | 0.0040 ± 0.0004 | 0.5 ± 0.1 | 0.007 ± 0.001 | 0.03 ± 0.01 |
| Ferrocene acetonitrile | 0.020 ± 0.003 | 0.010 ± 0.002 | 0.07 ± 0.02 | 0.0014 ± 0.0001 | 0.14 ± 0.05 |
| Mediator/Biomaterial | Test Function | IC50 of the Toxicant, mg/dm3 | Ref | |||||
|---|---|---|---|---|---|---|---|---|
| Pb2+ | Cd2+ | Cu2+ | Zn2+ | Phenol | p-Nitrophenol | |||
| Ferrocene/ S. cerevisiae | Microbial metabolic activity decrease | 6.1 | – * | 2.7 | 17.7 | 1.8 | – * | This work |
| Ferrocene/ E. coli | 0.8 | 8.9 | 47.6 | 46.4 | 17.6 | 5.8 | This work | |
| Ferrocene/ Ps. Veronii | 169.8 | – * | 286.3 | – * | 13.5 | – * | This work | |
| Ferrocene/ P. yeei, E. coli association | 7.3 | 6.6 | 23.8 | 2.3 | 8.1 | 29.2 | This work | |
| Ferrocene/ P. yeei | 9.9 | 18.2 | 21.1 | 47.5 | 9.9 | 2.1 | [27] | |
| p-Benzoquinone/ E. coli, B. subtilis, S. cerevisiae | – * | 20.5 | 16.5 | – * | – * | – * | [17] | |
| Menadione and potassium hexacyanoferrate(III)/ S. cerevisiae | 34.6 | 13.9 | 10.1 | – * | 44.5 | – * | [18] | |
| Thionine/ E. coli | 34.4 | 36.2 | 20.2 | 53.2 | – * | – * | [19] | |
| p-Benzoquinone/ Psychrobacter sp. isolated from activated sludge | 110 | 47.3 | 2.6 | 10.9 | – * | – * | [20] | |
| Potassium hexacyanoferrate(III)/ activated sludge | – * | 13.4 | 19.8 | 1.19 | – * | – * | [21] | |
| Duckweed (Lemna) | Inhibition of duckweed growth rate | 5.5 | 0.33 | 0.33 | 0.9 | – * | – * | [15] |
| Vibrio fischeri | Decrease of microbial bioluminescence intensity | 36.0 | 52.5 | 34.4 | 4.64 | – * | – * | [15] |
| Chlorella vulgaris | Evolution of oxygen in the gas phase | 0.476 | 0.301 | – * | – * | – * | – * | [16] |
3.3. Toxic Effects of Model Solutions on Characteristics of Ferrocene–P. yeei, Ferrocene–E. coli, and Ferrocene–S. cerevisiae Biosensor Systems
3.4. Characteristics of Developed Systems
3.5. Determination of Integral Toxicity of Wastewater Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rekhi, H.; Rani, S.; Sharma, N. A review on recent applications of high-performance liquid chromatography in metal determination and speciation analysis. Crit. Rev. Anal. Chem. 2017, 47, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Talaee, M.; Lorestani, B.; Ramezani, M.; Cheraghi, M.; Jameh-Bozorgi, S. Tandem dispersive liquid–liquid microextraction coupled with micro-sampling flame atomic absorption spectrometry for rapid determination of lead (II) and cadmium (II) ions in environmental water samples. Int. J. Env. Anal. Chem. 2019, 99, 1235–1246. [Google Scholar] [CrossRef]
- Javed, M.; Usmani, N. Impact of heavy metal toxicity on hematology and glycogen status of fish: A review. Proc. Nat. Acad. Sci. India Sect. B Biol. Sci. 2014, 85, 889–900. [Google Scholar] [CrossRef]
- Van der Schalie, W.H.; Shedd, T.R.; Knechtges, P.L.; Widder, M.W. Using higher organisms in biological early warning systems for real-time toxicity detection. Biosens. Bioelectron. 2001, 16, 457–465. [Google Scholar] [CrossRef]
- Robinson, A.A.; Belden, J.B.; Lydy, M.J. Toxicity of fluoroquinolone antibiotics to aquatic organisms. Environ. Toxicol. Chem. 2005, 24, 423–430. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, S.; Upreti, N.; Sharma, K.P. Monitoring toxicity of an azo dye methyl red and a heavy metal Cu using plant and animal bioassays. Toxicol. Environ. Chem. 2009, 91, 109–120. [Google Scholar] [CrossRef]
- Nelson, S.M.; Roline, R.A. Evaluation of the sensitivity of rapid toxicity tests relative to daphnid acute lethality tests. Bull. Environ. Contam. Toxicol. 1998, 60, 292–299. [Google Scholar] [CrossRef]
- Martins, J.; Oliva Teles, L.; Vasconcelos, V. Assays with Daphnia magna and Danio rerio as alert systems in aquatic toxicology. Environ. Int. 2007, 33, 414–425. [Google Scholar] [CrossRef]
- Girotti, S.; Ferri, E.N.; Fumo, M.G.; Maiolini, E. Monitoring of environmental pollutants by bioluminescent bacteria. Anal. Chim. Acta 2008, 608, 2–29. [Google Scholar] [CrossRef]
- Parvez, S.; Venkataraman, C.; Mukherji, S. A review on advantages of implementing luminescence inhibition test (Vibrio fischeri) for acute toxicity prediction of chemicals. Environ. Int. 2006, 32, 265–268. [Google Scholar] [CrossRef]
- Kim, Y.M.; Park, D.; Lee, D.S.; Park, J.M. Inhibitory effects of toxic compounds on nitrification process for cokes wastewater treatment. J. Hazard. Mat. 2008, 152, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.H.A.; Van Ginkel, W.V.; Hussein, M.M.A.; Abskharon, R.; Oh, S.-E. Toxicity assessment using different bioassays and microbial biosensors. Environ. Int. 2016, 92, 106–118. [Google Scholar] [CrossRef] [PubMed]
- ISO 11348-1:2007/AMD 1:2018; Water Quality–Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent Bacteria Test)–Part 1: Method Using Freshly Prepared Bacteria. ISO: Geneva, Switzerland, 2017.
- Woutersen, M.; Van der Gaag, B.; Boakye, A.A.; Mink, J.; Marks, R.S.; Wagenvoort, A.J.; Ketelaars, H.A.M.; Brouwer, B.; Heringa, M.B. Development and validation of an on-line water toxicity sensor with immobilized luminescent bacteria for on-line surface water monitoring. Sensors 2017, 17, 2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizullah, A.; Häder, D.P. A comparison of commonly used and commercially available bioassays for aquatic ecosystems. In Bioassays; Elsevier: Amsterdam, The Netherlands, 2018; Volume 17, pp. 347–368. [Google Scholar]
- Eom, H.; Park, M.; Jang, A.; Kim, S.; Oh, S.-E. A simple and rapid algal assay kit to assess toxicity of heavy metal-contaminated water. Environ. Pollution. 2021, 269, 116–135. [Google Scholar] [CrossRef]
- Gao, G.; Qian, J.; Fang, D.; Yu, Y.; Zhi, J. Development of a mediated whole cell-based electrochemical biosensor for joint toxicity assessment of multi-pollutants using a mixed microbial consortium. Anal. Chim. Acta 2016, 924, 21–28. [Google Scholar] [CrossRef]
- Gao, G.; Fang, D.; Yu, Y.; Wu, L.; Wang, Y.; Zhi, J. A double-mediator based whole cell electrochemical biosensor for acute biotoxicity assessment of wastewater. Talanta 2017, 167, 208–216. [Google Scholar] [CrossRef]
- Fang, D.; Gao, G.; Shen, J.; Yu, Y.; Zhi, J. A reagentless electrochemical biosensor based on thionine wrapped E. coli and chitosan-entrapped carbon nanodots film modified glassy carbon electrode for wastewater toxicity assessment. Electrochim. Acta 2016, 22, 303–311. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Wang, X.; Wu, Z.; Yang, L.; Xia, S.; Chen, L.; Zhao, J. p-Benzoquinone-mediated amperometric biosensor developed with Psychrobacter sp. for toxicity testing of heavy metals. Biosens. Bioelectron. 2013, 41, 557–562. [Google Scholar] [CrossRef]
- Ma, H.; Yong, D.; Kim, H.; Zhang, Z.; Ma, S.; Han, X. A ferricyanide-mediated activated sludge bioassay for determination of the toxicity of water. Electroanalysis 2015, 28, 580–587. [Google Scholar] [CrossRef]
- Fang, D.; Gao, G.; Yang, Y.; Wang, Y.; Gao, L.; Zhi, J. Redox mediator-based microbial biosensors for acute water toxicity assessment: A critical review. Chem. Electro. Chem. 2020, 7, 2513–2526. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, D.; Liu, Y.; Liu, R.; Wang, X.; Yu, Y.; Zhi, J. Problems analysis and new fabrication strategies of mediated electrochemical biosensors for wastewater toxicity assessment. Biosens. Bioelectron. 2018, 108, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, Y.; Han, X.; Chang, X. The fabrication and the use of immobilized cells as test organisms in a ferricyanide-based toxicity biosensor. Environ. Toxicol. Chem. 2017, 3, 329–335. [Google Scholar] [CrossRef]
- Qian, J.; Li, J.; Fang, D.; Yu, Y.; Zhi, J. A disposable biofilm-modified amperometric biosensor for the sensitive determination of pesticide biotoxicity in water. RSC Adv. 2014, 98, 55473–55482. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Bu, Y.; Yan, X.; Chen, J.; Zhao, J. Direct toxicity assessment of copper (II) ions to activated sludge process using a p-benzoquinone-mediated amperometric biosensor. Sens. Actuat. B Chemical. 2015, 208, 554–558. [Google Scholar] [CrossRef]
- Kharkova, A.S.; Arlyapov, V.A.; Turovskaya, A.D.; Shvets, V.I.; Reshetilov, A.N. A mediator microbial biosensor for assaying general toxicity. Enzyme Microb. Technol. 2020, 132, 109435. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, Y.; Wang, Y.; Qian, J.; Zhi, J. The benzoquinone-mediated electrochemical microbial biosensor for water biotoxicity assay. Electrochim. Acta 2013, 97, 52–57. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Chen, Y.; Wang, Y.; Shao, P.; Liu, R.; Gao, G.; Zhi, J. A portable instrument for monitoring acute water toxicity based on mediated electrochemical biosensor: Design, testing and evaluation. Chemosphere 2020, 255, 126964. [Google Scholar] [CrossRef]
- Kharkova, A.S.; Arlyapov, V.A.; Turovskaya, A.D.; Avtukh, A.N.; Starodumova, I.P.; Reshetilov, A.N. Mediator BOD biosensor based on cells of microorganisms isolated from activated sludge. Appl. Biochem. Microbiol. 2019, 55, 189–197. [Google Scholar] [CrossRef]
- ISO 8692; Water Quality–Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae. 3rd ed. ISO: Geneva, Switzerland, 2012.
- ISO 10872; Water and Soil Quality–Determination of the Toxic Effect of Sediment and Soil Samples on Growth, Fertility and Reproduction of Caenorhabditis elegans (Nematoda). ISO: Geneva, Switzerland, 2020.
- Arlyapov, V.A.; Yudina NYu Asulyan, L.D.; Kamanina, O.A.; Alferov, S.V.; Shumsky, A.N.; Machulin, A.V.; Alferov, V.A.; Reshetilov, A.N. Registration of BOD using Paracoccus yeei bacteria isolated from activated sludge. 3 Biotech 2020, 10, 207. [Google Scholar] [CrossRef]
- Nam, I.-H.; Chang, Y.-S.; Hong, H.-B.; Lee, Y.-E. A novel catabolic activity of Pseudomonas veronii in biotransformation of pentachlorophenol. Appl. Microbiol. Biotechnol. 2003, 62, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.L.; Ceretti, H.M.; Daneil, M.A.; Ramirez, S.A.M.; Zalts, A. Cadmium, zinc and copper biosorption mediated by Pseudomonas veronii 2E. Biores. Technol. 2008, 99, 5574–5581. [Google Scholar] [CrossRef] [PubMed]
- Goffeau, A.; Barrell, B.G.; Bussey, H.; Davis, R.W.; Dujon, B.; Feldmann, H.; Galibert, F.; Hoheisel, J.D.; Jacq, C.; Johnston, M.; et al. Life with 6000 genes. Science 1996, 274, 546–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strandberg, G.W.; Shumate, S.E.; Parrott, J.R. Microbial cells as biosorbents for heavy metals: Accumulation of uranium by Saccharomyces cerevisiae and Pseudomonas aeruginosa. Appl. Environ. Microbiol. 1981, 41, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, M.; Riedel, K.; Adler, K.; Kunze, G. Amperometric measurement of copper ions with a deputy substrate using a novel Saccharomyces cerevisiae sensor. Biosens. Bioelectron. 2000, 15, 211–219. [Google Scholar] [CrossRef]
- Ikeda, T.; Kurosaki, T.; Takayama, K.; Kano, K. Measurements of oxidoreductase-like activity of intact bacterial cells by an amperometric method using a membrane-coated electrode. Anal. Chem. 1996, 68, 192–198. [Google Scholar] [CrossRef]
- Yudina NYu Arlyapov, V.A.; Chepurnova, M.A.; Alferov, S.V.; Reshetilov, A.N. A yeast co-culture-based biosensor for determination of wastewater contamination levels. Enzyme Microb. Technol. 2015, 78, 46–53. [Google Scholar] [CrossRef]
- Fairbanks, V.F. Copper sulfate-induced hemolytic anemia: Inhibition of glucose-6-phosphate dehydrogenase and other possible etiologic mechanisms. Arch. Int. Med. 1967, 120, 428–432. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Gataullina, M.O.; Lyashchenko, M.S. Physicochemical and catalytic properties of NAD+-dependent malate dehydrogenase isoforms from maize mesophyll. Appl. Biochem. Microbiol. 2016, 52, 366–370. [Google Scholar] [CrossRef]
- Naučienė, Z.; Mildažienė, V.; Banienė, R. Interactions of cadmium and copper ions with complex I of the respiratory chain in rat liver mitochondria. Ekologija 2002, 2, 18–21. [Google Scholar]
- Mufti, A.R.; Burstein, E.; Duckett, C.S. XIAP: Cell death regulation meets copper homeostasis. Arch. Biochem. Biophys. 2007, 463, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poisa, L.; Adamovics, A. Hemp (Cannabis sativa L.) as an environmentally friendly energyplant. Rigas Tehn. Universit. Zinatn. Raksti. 2010, 5, 80. [Google Scholar] [CrossRef]
- Zhmaeva, E.V.; Bychkov, P.V.; Shekhovtsova, T.N. Enzymatic determination of mercury (II) and methylmercury using alcohol dehydrogenases of different origin. Vestn. MGU Khim. 2002, 6, 404. [Google Scholar]
- Alunni, S.; Cipiciani, A.; Fioroni, G.; Ottavi, L. Mechanisms of inhibition of phenylalanine ammonia-lyase by phenol inhibitors and phenol/glycine synergistic inhibitors. Arch. Biochem. Biophys. 2003, 412, 170–175. [Google Scholar] [CrossRef]






| Growth Phase | P. yeei VKM B-3302 | E. coli K802 | S. cerevisiae VKM Y-1173 | Ps. veronii DSM 11331T |
|---|---|---|---|---|
| Lag phase, h | 0–2 | 0–2 | 0–2 | 0–6 |
| Exponential phase, h | 2–4 | 2–4 | 2–4 | 6–12 |
| Linear growth phase, h | 4–16 | 4–10 | 4–8 | 12–20 |
| Transition phase (growth slowdown phase), h | 16–24 | 10–20 | 8–17 | 20–36 |
| Stationary phase, h | 24–30 | 20–30 | 17–30 | 36–52 |
| Specific growth rate, h−1 | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.71 ± 0.04 | 0.265 |
| Biomaterial/Mediator | Toxicant Solution | Toxicant Concentration at which the Metabolic Activity of Cells Decreases by 20%, IC20, mg/dm3 | Reversibility Degree, % |
|---|---|---|---|
| P. yeei–ferrocene | Cu2+ | 10 | 96 ± 4 |
| Phenol | 4 | 96 ± 2 | |
| E. coli–ferrocene | Cu2+ | 24 | 97 ± 2 |
| Phenol | 8 | 94 ± 4 | |
| S. cerevisiae–ferrocene | Cu2+ | 1.5 | 94 ± 3 |
| Phenol | 1 | 93 ± 5 | |
| P. yeei/E.coli association–ferrocene | Zn2+ | 2.3 | 96 ± 2 |
| Phenol | 8.1 | 93 ± 4 |
| Biomaterial/ Mediator | Toxicant | KM, mmol/dm3 | KMI mmol/dm3 | rmax, μA | rmaxI, μA | Inhibition Type |
|---|---|---|---|---|---|---|
| P. yeei/ ferrocene | Cu2+ | 8 ± 2 | 13 ± 2 | 8.1 ± 0.4 | 8.1 ± 0.3 | Competitive |
| Phenol | 9 ± 2 | 6 ± 1 | 5.3 ± 0.3 | 4.5 ± 0.3 | Mixed | |
| E. coli/ ferrocene | Cu2+ | 2.1 ± 0.9 | 0.5 ± 0.05 | 2.5 ± 0.3 | 1.97 ± 0.03 | Mixed |
| Phenol | 2.1 ± 0.5 | 1.0 ± 0.3 | 2.9 ± 0.2 | 2.3 ± 0.2 | Mixed | |
| S. cerevisiae/ ferrocene | Cu2+ | 1.9 ± 0.1 | 1.6 ± 0.2 | 1.3 ± 0.1 | 0.77 ± 0.04 | Mixed |
| Phenol | 3.2 ± 0.9 | 5 ± 2 | 1.0 ± 0.2 | 1.1 ± 0.4 | Competitive |
| Biomaterial/Mediator | Toxicant | Long-Time Stability, Days | Operational Stability, % | Reproducibility, % |
|---|---|---|---|---|
| P. yeei/ferrocene | Cu2+ | 9 | 4.9 | 4.5 |
| Phenol | 10 | 5.3 | 4.8 | |
| E. coli/ferrocene | Cu2+ | 3 | 6.92 | 7 |
| Phenol | 3 | 10.81 | 9 | |
| S. cerevisiae/ferrocene | Cu2+ | 5 | 6.9 | 4.9 |
| Phenol | 5 | 11.5 | 5.1 | |
| P. yeei, E. coli/ferrocene | Cu2+ | 4 | 7.3 | 5.2 |
| Phenol | 4 | 10.3 | 9.6 |
| Method of Analysis | TDF of Analyzed Samples | ||
|---|---|---|---|
| Sample No 1 | Sample No 2 | Sample No 3 | |
| Chlorella vulgaris biotesting | 56-fold (highly toxic) | 21-fold (toxic) | 7-fold (mid toxic) |
| Ferrocene–E. coli biosensor | 63-fold (highly toxic) | 16-fold (toxic) | 5-fold (mid toxic) |
| Ferrocene–P. yeei biosensor | 25-fold (toxic) | 6-fold (mid toxic) | 2-fold (low toxic) |
| Ferrocene–S. cerevisiae biosensor | 45-fold (highly toxic) | 60-fold (highly toxic) | 32-fold (highly toxic) |
| Ferrocene–E. coli/P. yeei biosensor | 60-fold (highly toxic) | 19-fold (toxic) | 8-fold (toxic) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharkova, A.; Arlyapov, V.; Medvedeva, A.; Lepikash, R.; Melnikov, P.; Reshetilov, A. Mediator Microbial Biosensor Analyzers for Rapid Determination of Surface Water Toxicity. Sensors 2022, 22, 8522. https://doi.org/10.3390/s22218522
Kharkova A, Arlyapov V, Medvedeva A, Lepikash R, Melnikov P, Reshetilov A. Mediator Microbial Biosensor Analyzers for Rapid Determination of Surface Water Toxicity. Sensors. 2022; 22(21):8522. https://doi.org/10.3390/s22218522
Chicago/Turabian StyleKharkova, Anna, Vyacheslav Arlyapov, Anastasia Medvedeva, Roman Lepikash, Pavel Melnikov, and Anatoly Reshetilov. 2022. "Mediator Microbial Biosensor Analyzers for Rapid Determination of Surface Water Toxicity" Sensors 22, no. 21: 8522. https://doi.org/10.3390/s22218522