3D Printing Techniques and Their Applications to Organ-on-a-Chip Platforms: A Systematic Review
Abstract
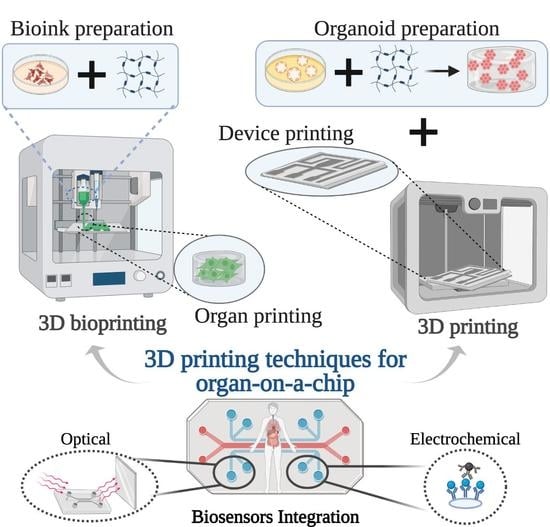
:1. Introduction
2. Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Data Analysis
3. Results and Discussion
3.1. 3D Printing Techniques
3.2. 3D Bioprinting Techniques
3.2.1. Nervous-System-on-a-Chip
3.2.2. Multi-Organ-on-a-Chip
3.2.3. Vascularized Tissue-on-a-Chip
3.2.4. Liver-on-a-Chip
3.2.5. Renal Tubule-on-a-Chip
3.2.6. Vessel-on-a-Chip
3.2.7. Myocardium-on-a-Chip
3.2.8. Gut-on-a-Chip
3.2.9. Thrombosis-on-a-Chip
3.2.10. Tumor Array-on-a-Chip
3.2.11. Placenta-on-a-chip
3.3. New Approaches and Other Applications of 3D (Bio)Printing to Fabricate OoC Platforms without Specifying the Target Organ
4. Other Challenges in Organs-on-Chip Devices: Sensors Integration
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, Y.; Ih Ahn, S.; Kim, Y.T. Organs-On-Chips; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 1–3, ISBN 9780128051443. [Google Scholar]
- Chan, C.Y.; Huang, P.-H.; Guo, F.; Ding, X.; Kapur, V.; Mai, J.D.; Yuen, P.K.; Huang, T.J. Accelerating drug discovery via organs-on-chips. Lab Chip 2013, 13, 4697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jodat, Y.A.; Kang, M.G.; Kiaee, K.; Kim, G.J.; Martinez, F.H.; Rosenkranz, A.; Bae, H.; Shin, S.R.; Program, M.L.; Biotechnology, R. Human-Derived Organ-on-a-Chip for Personalized Drug Development. Curr. Pharm. Des. 2019, 24, 5471–5486. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Devarasetty, M.; Forsythe, S.; Atala, A.; Soker, S. A reductionist metastasis-on-a-chip platform for in vitro tumor progression modeling and drug screening. Biotechnol. Bioeng. 2016, 113, 2020–2032. [Google Scholar] [CrossRef] [Green Version]
- Saglam-Metiner, P.; Gulce-Iz, S.; Biray-Avci, C. Bioengineering-inspired three-dimensional culture systems: Organoids to create tumor microenvironment. Gene 2019, 686, 203–212. [Google Scholar] [CrossRef]
- Bhise, N.S.; Manoharan, V.; Mi, S.; Yi, X.; Jun, L.; Ong, Y.; Islam, A.; Shashkova, S.; Leake, M.C. Design and fabrication of a scalable liver-lobule- on-a-chip microphysiological platform. Int. Soc. Biofabr. 2017, 9, 015014. [Google Scholar]
- Carvalho, V.; Maia, I.; Souza, A.; Ribeiro, J.; Costa, P.; Puga, H.; Teixeira, S.F.C.F.; Lima, R.A. In vitro stenotic arteries to perform blood analogues flow visualizations and measurements: A Review. Open Biomed. Eng. J. 2020, 14, 87–102. [Google Scholar] [CrossRef]
- Rajan, S.A.P.; Aleman, J.; Wan, M.M.; Pourhabibi Zarandi, N.; Nzou, G.; Murphy, S.; Bishop, C.E.; Sadri-Ardekani, H.; Shupe, T.; Atala, A.; et al. Probing prodrug metabolism and reciprocal toxicity with an integrated and humanized multi-tissue organ-on-a-chip platform. Acta Biomater. 2020, 106, 124–135. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Sousa, P.C.; Gaspar, J.; Bañobre-López, M.; Lima, R.; Minas, G. Organ-on-a-Chip: A Preclinical Microfluidic Platform for the Progress of Nanomedicine. Small 2020, 16, 2003517. [Google Scholar] [CrossRef]
- Lee, H.; Cho, D.W. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip 2016, 16, 2618–2625. [Google Scholar] [CrossRef] [Green Version]
- Hiller, T.; Berg, J.; Elomaa, L.; Röhrs, V.; Ullah, I.; Schaar, K.; Dietrich, A.C.; Al-Zeer, M.A.; Kurtz, A.; Hocke, A.C.; et al. Generation of a 3D liver model comprising human extracellular matrix in an alginate/gelatin-based bioink by extrusion bioprinting for infection and transduction studies. Int. J. Mol. Sci. 2018, 19, 3129. [Google Scholar] [CrossRef] [Green Version]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Mao, M.; He, J.; Lu, Y.; Li, T.; Zhou, W.; Li, D. Leaf-templated, microwell-integrated microfluidic chips for high- throughput cell experiments. Biofabrication 2018, 10, 25008. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lim, J.; Choi, M.; Chung, M.; Jeon, N.L. From microchannels to microphysiological systems: Development of application specific devices. Microelectron. Eng. 2018, 202, 9–18. [Google Scholar] [CrossRef]
- Ozbolat, V.; Dey, M.; Ayan, B.; Ozbolat, I.T. Extrusion-based printing of sacrificial Carbopol ink for fabrication of microfluidic devices. Biofabrication 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Schneider, S.; Loskill, P. High-throughput organ-on-a-chip systems: Current status and remaining challenges. Curr. Opin. Biomed. Eng. 2018, 6, 33–41. [Google Scholar] [CrossRef]
- Catarino, S.O.; Rodrigues, R.O.; Pinho, D.; Miranda, J.M.; Minas, G.; Lima, R. Blood Cells Separation and Sorting Techniques of Passive Microfluidic Devices: From Fabrication to Applications. Micromachines 2019, 10, 593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.-J.; Kaji, H. Modeling angiogenesis with micro- and nanotechnology. Lab Chip 2017, 17, 4186–4219. [Google Scholar] [CrossRef]
- Faustino, V.; Catarino, S.O.; Lima, R.; Minas, G. Biomedical microfluidic devices by using low-cost fabrication techniques: A review. J. Biomech. 2016, 49, 2280–2292. [Google Scholar] [CrossRef] [Green Version]
- Miri, A.K.; Mirzaee, I.; Hassan, S.; Oskui, M.; Nieto, D.; Shrike, Y. Effective bioprinting resolution in tissue model fabrication. Lab Chip 2019, 19, 2019–2037. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mao, Q.; Li, X.; Yin, J.; Wang, Y.; Fu, J.; Huang, Y. High-fidelity and high-efficiency additive manufacturing using tunable pre-curing digital light processing. Addit. Manuf. 2019, 30, 100889. [Google Scholar] [CrossRef]
- Miri, A.; Mostafavi, E.; Khorsandi, D.; Hu, S.-K.; Malpica, M.; Khademhosseini, A. Bioprinters for organs-on-chips. Int. Soc. Biofabr. 2019, 11, 042002. [Google Scholar] [CrossRef]
- Ding, H.; Illsley, N.P.; Chang, R.C. 3D Bioprinted GelMA Based Models for the Study of Trophoblast Cell Invasion. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; Mcclinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2019, 2020, 119536. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.A.; Mollica, P.A.; Johnson, G.D.; Ogle, R.C.; Bruno, R.D.; Sachs, P.C. Accessible bioprinting: Adaptation of a low-cost 3D-printer for precise cell placement and stem cell differentiation. Biofabrication 2016, 8, 025017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gungor-ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Dokmeci, M.R.; Sciences, N.; Angeles, L.; Arabia, S.; Angeles, L. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [Green Version]
- Ozbolat, I.T.; Moncal, K.K.; Gudapati, H. Evaluation of bioprinter technologies. Addit. Manuf. 2017, 13, 179–200. [Google Scholar] [CrossRef] [Green Version]
- Ambhorkar, P.; Rakin, R.H.; Wang, Z.; Kumar, H.; Kim, K. Biofabrication strategies for engineering heterogeneous artificial tissues. Addit. Manuf. 2020, 36, 101459. [Google Scholar] [CrossRef]
- Deo, K.A.; Singh, K.A.; Peak, C.W.; Alge, D.L.; Gaharwar, A.K. Bioprinting 101: Design, Fabrication, and Evaluation of Cell-Laden 3D Bioprinted Scaffolds. Tissue Eng. Part A 2020, 26, 318–338. [Google Scholar] [CrossRef]
- Yi, H.; Lee, H.; Cho, D. 3D Printing of Organs-On-Chips. Bioengineering 2017, 4, 10. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.; Gao, Q.; Wang, Y.; Zeng, J.; Zhao, H.; Sun, Y.; Shen, J.; Ramezani, H.; Fu, Z.; Liu, Z.; et al. Vessel-on-a-chip with Hydrogel-based Microfluidics. Small 2018, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.A.U.; Kim, Y.S.; Ali, M.; Lee, B.G.; Cho, Y.-J.; Choi, K.H. A lung cancer-on-chip platform with integrated biosensors for physiological monitoring and toxicity assessment. Biochem. Eng. J. 2020, 155, 107469. [Google Scholar] [CrossRef]
- Cho, S.; Islas-Robles, A.; Nicolini, A.M.; Monks, T.J.; Yoon, J.-Y. In situ, dual-mode monitoring of organ-on-a-chip with smartphone-based fluorescence microscope. Biosens. Bioelectron. 2016, 86, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Lantada, A.D.; Pfleging, W.; Besser, H.; Guttmann, M.; Wissmann, M.; Plewa, K.; Smyrek, P.; Piotter, V.; García-Ruíz, J.P. Research on the methods for the mass production of multi-scale organs-on-chips. Polymers 2018, 10, 1238. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, J.; Ghadiri, M.; Shanmugavel, M.; Razavi Bazaz, S.; Vasilescu, S.; Ding, L.; Ebrahimi Warkiani, M. A rapidly prototyped lung-on-a-chip model using 3D-printed molds. Organs-Chip 2019, 1, 100001. [Google Scholar] [CrossRef]
- Kalaskar, D.M. 3D Printing in Medicine, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780081007174. [Google Scholar]
- Zhou, G.; Liu, W.; Zhang, Y.; Gu, W.; Li, M.; Lu, C.; Zhou, R.; Che, Y.; Lu, H.; Zhu, Y.; et al. Application of three-dimensional printing in interventional medicine. J. Interv. Med. 2020, 3, 1–16. [Google Scholar] [CrossRef]
- Datta, P.; Ayan, B.; Ozbolat, I.T. Bioprinting for Vascular and Vascularized Tissue Biofabrication. Acta Biomater. 2017, 51, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, B.N.; Lancaster, K.Z.; Hogue, I.B.; Meng, F.; Kong, Y.L.; Enquist, L.W.; McAlpine, M.C. 3D printed nervous system on a chip. Lab Chip 2016, 16, 1393–1400. [Google Scholar] [CrossRef]
- Bowser, D.A.; Moore, M.J. Biofabrication of neural microphysiological systems using magnetic spheroid bioprinting. Int. Soc. Biofabr. 2020, 12, 015002. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Murphy, S.V.; Devarasetty, M.; Mead, I.; Kang, H.; Seol, Y.; Zhang, Y.S.; Shin, S.; Zhao, L.; Aleman, J.; et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci. Rep. 2017, 7, 8837. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Homan, K.A.; Skylar-scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef] [Green Version]
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.S.; Shin, S.R.; Calzone, G.; et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101. [Google Scholar] [CrossRef]
- Lee, H.; Chae, S.; Kim, J.Y.; Han, W.; Kim, J.; Choi, Y. Cell-printed 3D liver-on-a-chip possessing a liver microenvironment and biliary system. Int. Soc. Biofabr. 2019, 11, 025001. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; Choi, Y.; Cho, D.W. Application of Gelatin Bioinks and Cell-Printing Technology to Enhance Cell Delivery Capability for 3D Liver Fibrosis-on-a-Chip Development. ACS Biomater. Sci. Eng. 2020, 6, 2469–2477. [Google Scholar] [CrossRef]
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci. Rep. 2016, 6, 34845. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Liu, Z.; Lin, Z.; Qiu, J.; Liu, Y.; Liu, A.; Xiang, M.; Chen, B.; Fu, J.; He, Y. 3D Bioprinting of Vessel-like Structures with Multi-level Fluidic Channels. ACS Biomater. Sci. Eng. 2017. [Google Scholar] [CrossRef]
- Abudupataer, M.; Chen, N.; Yan, S.; Alam, F.; Shi, Y.; Wang, L.; Lai, H.; Li, J.; Zhu, K.; Wang, C. Bioprinting a 3D vascular construct for engineering a vessel-on-a-chip. Biomed. Microdevices 2020, 22, 10. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Aleman, J.; Arneri, A.; Bersini, S.; Shin, S.-R.S.R.; Dokmeci, M.R.; Khademhosseini, A.; Arabia, S.; Zhu, K.; Goli-Malekabadi, Z.; et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrotra, S.; De Melo, B.A.G.; Hirano, M.; Keung, W.; Li, R.A.; Mandal, B.B.; Shin, S.R. Nonmulberry Silk Based Ink for Fabricating Mechanically Robust Cardiac Patches and Endothelialized Myocardium-on-a-Chip Application. Adv. Mater. 2020, 30, 1907436. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, G. Intestinal Villi Model with Blood Capillaries Fabricated Using Collagen-Based Bioink and Dual-Cell-Printing Process. ACS Appl. Mater. Interfaces 2018, 10, 41185–41196. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Davoudi, F.; Walch, P.; Manbachi, A.; Luo, X.; Dell’Erba, V.; Miri, A.K.; Albadawi, H.; Arneri, A.; Li, X.; et al. Bioprinted thrombosis-on-a-chip. Lab Chip 2016, 16, 4097–4105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, M.; Gao, Q.; Fu, J.; Chen, Z.; He, Y. Bioprinting of novel 3D tumor array chip for drug screening. Bio-Design Manuf. 2020, 3, 175–188. [Google Scholar] [CrossRef]
- Kuo, C.; Eranki, A.; Placone, J.K.; Rhodes, K.R.; Aranda-espinoza, H.; Fernandes, R.; Fisher, J.P.; Kim, P.C.W. Development of a 3D Printed, Bioengineered Placenta Model to Evaluate the Role of Trophoblast Migration in Preeclampsia. ACS Biomater. Sci. Eng. 2016, 2, 1817–1826. [Google Scholar] [CrossRef]
- Deng, J.; Wei, W.; Chen, Z.; Lin, B.; Zhao, W.; Luo, Y.; Zhang, X. Engineered liver-on-a-chip platform to mimic liver functions and its biomedical applications: A review. Micromachines 2019, 10, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.; Shevchuk, M.; Opfermann, J.; Guo, T.; Fisher, J.P.; Kim, P.C.W. Trophoblast-Endothelium Signaling Involves Angiogenesis and Apoptosis in a Dynamic Bioprinted Placenta Model. Biotechnol. Bioeng. 2020, 116, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Grix, T.; Ruppelt, A.; Thomas, A.; Amler, A.; Noichl, B.P.; Lauster, R.; Kloke, L. Bioprinting Perfusion-Enabled Liver Equivalents for Advanced Organ-on-a-Chip Applications. Genes 2018, 9, 176. [Google Scholar] [CrossRef] [Green Version]
- Bertassoni, L.E.; Cardoso, J.C.; Manoharan, V.; Cristino, A.L.; Bhise, N.S.; Araujo, W.A.; Zorlutuna, P.; Vrana, N.E.; Ghaemmaghami, A.M.; Dokmeci, M.R.; et al. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication 2014, 6, 024105. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.; Almeida, E.; Guvendiren, M. 3D bioprinting of complex channels within cell-laden hydrogels. Acta Biomater. 2019, 95, 214–224. [Google Scholar] [CrossRef]
- Rocca, M.; Fragasso, A.; Liu, W.; Heinrich, M.A.; Zhang, Y.S. Embedded Multimaterial Extrusion Bioprinting. SLAS Technol. 2018, 23, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Patrício, S.; Sousa, L.; Correia, T.; Gaspar, V.; Pires, L.; Oliveira, J.; Mano, J. Freeform 3D Printing using a Continuous Viscoelastic Supporting Matrix. Int. Soc. Biofabr. 2020, 12, 035017. [Google Scholar] [CrossRef]
- Günther, K.; Sonntag, F.; Lasagni, A.F.; Moritzer, E.; Hirsch, A.; Klotzbach, U.; Lasagni, A.F. Universal micromachining platform and basic technologies for the manufacture and marking of microphysiological systems. Micromachines 2017, 8, 246. [Google Scholar] [CrossRef] [Green Version]
- Xiong, R.; Chai, W.; Huang, Y. Laser printing-enabled direct creation of cellular heterogeneity in lab-on-a-chip devices. Lab Chip 2019, 19, 1644–1656. [Google Scholar] [CrossRef]
- Sonntag, F.; Schmieder, F.; Ströbel, J.; Grünzner, S.; Busek, M.; Günther, K.; Steege, T.; Polk, C.; Klotzbach, U. Universal lab-on-a-chip platform for complex, perfused 3D cell cultures. Microfluid. BioMEMS Med. Microsyst. XIV 2016, 9705, 970516. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Miranda, C.C.; Cabral, J.M.S. Modeling the Human Body on Microfluidic Chips. Trends Biotechnol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kilic, T.; Navaee, F.; Stradolini, F.; Renaud, P.; Carrara, S. Organs-on-chip monitoring: Sensors and other strategies. Microphysiolo. Syst. 2018, 1. [Google Scholar] [CrossRef]
- Kieninger, J.; Weltin, A.; Flamm, H.; Urban, G.A. Microsensor systems for cell metabolism–From 2D culture to organ-on-chip. Lab Chip 2018, 18, 1274–1291. [Google Scholar] [CrossRef] [Green Version]
- Rebelo, R.; Barbosa, A.I.; Caballero, D.; Kwon, I.K.; Oliveira, J.M.; Kundu, S.C.; Reis, R.L.; Correlo, V.M. 3D biosensors in advanced medical diagnostics of high mortality diseases. Biosens. Bioelectron. 2019, 130, 20–39. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Hernández, J.E.; Villalba-Rodríguez, A.M.; Romero-Castillo, K.D.; Aguilar-Aguila-Isaías, M.A.; García-Reyes, I.E.; Hernández-Antonio, A.; Ahmed, I.; Sharma, A.; Parra-Saldívar, R.; Iqbal, H.M.N. Organs-on-a-chip module: A review from the development and applications perspective. Micromachines 2018, 9, 536. [Google Scholar] [CrossRef] [Green Version]
- Ligon, S.C.; Liska, R.; Gurr, M.; Mu, R.; Gmbh, H.B.F.D.; Bleiche, A.D.R.; D-, L. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, A.; Lafont, U.; Hołyńska, M.; Semprimoschnig, C. Additive manufacturing—A review of 4D printing and future applications. Addit. Manuf. 2018, 24, 606–626. [Google Scholar] [CrossRef]




| Device | Printing Method | Application | Main Observations | Ref. | |
|---|---|---|---|---|---|
| Vessel-on-a-chip | - | Produce molds with diverse forms of channels. |  Reprinted with permission from ref. [32]. Copyright 2018 John Wiley and Sons. | A simple and cytocompatible approach was developed for fabricating hydrogel-based user-defined chips, suitable for the growth of organ or vascularized tissue models. | [32] |
| Lung cancer-on-a-chip | Inkjet | 3D-printed chip holder and elastomeric microfluidic channels and microfluidic connectors for cell culture media routing on the higher part of the glass. |  Reprinted with permission from ref. [33]. Copyright 2020 Elsevier. | This lung cancer-on-chip system, includes integrated biosensors for real-time monitoring of physiological events, can be used with any organ tissue or monolayer micro-tumor models for on-chip toxicity studies. | [33] |
| Metastasis-on-a-Chip | Plaster-based 3D printing | 3D-printed inverted chamber/channel structures as molds. |  Reprinted with permission from ref. [4]. Copyright 2016 John Wiley and Sons. | This system supports some aspects of the phenomena of metastasis, allowing to study the translocation of metastatic tumor cells from the primary tissue site to the downstream tissue site. | [4] |
| Vessel-on-a-chip | Extrusion-based 3D printing | 3D printing of channel prototypes with carbopol gel |  | It is presented a highly affordable and practical approach in the manufacture of PDMS devices with closed fluid channels, which have great potential to reconstitute a human endothelium-on-a-chip | [15] |
| Kidney-on-a-chip | FDM | 3D-printed template for conventional soft lithography fabrication of PDMS-based OoC |  Reprinted with permission from ref. [34]. Copyright 2016 Elsevier. | It is demonstrated the application of a 3D-printed template and a common cutter machine to provide a simple and affordable fabrication of OoC. | [34] |
| Multi-Organ-On-a-Chip | Laser SLA with epoxy resin | Produce master models for the chambers and channels of the fluidic device. |  Reprinted from ref. [35]. | This technology allows the design and rapid mass production of OoC devices. | [35] |
| Lung-on-a-chip | DLP | 3D-printed molds to manufacture a chip model with an open well design and with lower and upper layers to mimic the human lung. |  Reprinted from ref. [36]. Reprinted from ref. [36]. | The fabrication technique allows the chip to be fabricated in less than a day, and the molds can also be utilized for repeated PDMS casting. Therefore, the technique is robust, cost-effective, and simple. | [36] |
| OoC Platform | Printing Method | Schematic Representation | Cells Types | Bioink | Ref. |
|---|---|---|---|---|---|
| Nervous System-on-a-Chip | Micro-extrusion 3D printing strategies |  Reprinted with permission from ref. [40]. Copyright 2001 Royal Society of Chemistry. | Schwann cells, superior cervical ganglia and hippocampal neurons and epithelial cells | - | [40] |
| Central nervous system-on-a-chip | Magnetic bioprinting |  | Spinal cord cells | Neural spheroids | [41] |
| Multi-tissue OoC with liver, heart and lung organoids | Microextrusion bioprinting |  Reprinted from ref. [42]. | Hepatocyte; stellate; Kupffer iPS; lung fibroblasts, epithelial, and endothelial cells. | Spherical organoids with HA-gelatin hydrogel (liver) and fibrin-gelatin bioink (cardiac). | [42] |
| 3D vascularized tissue-on-a-chip | Microextrusion bioprinting |  Reprinted from ref. [43]. | hMSCs; hNDFs; HUVECs | Vascular ink (pluronic and thrombin) and cell-laden ink (gelatin–fibrin) | [43] |
| Liver-on-a-chip | Direct write bioprinter |  | HepG2/C3A cells | Hepatic spheroids and GelMA | [44] |
| Liver-on-a-chip | Microextrusion bioprinting |  Reprinted from ref. [10]. | HepG2; HUVECs. | Gelatin and liver dECM bioinks (collagen type 1) | [10] |
| Liver-on-a-chip | Microextrusion bioprinting |  | HepaRG and HUVECs | Gelatin and liver dECM bioinks (collagen type 1) | [45] |
| Liver Fibrosis-on-a-Chip | Microextrusion bioprinting |  Reprinted with permission from ref. [46]. Copyright 2020 American Chemical Society. | HepaRG, HUVECs and hepatic stellate cells | Gelatin and liver dECM bioinks (collagen type 1) | [46] |
| Convoluted 3D renal proximal tubules-on-a-chip | Extrusion custom-designed, multi-material 3D bioprinter |  Reprinted from ref. [47]. | PTECs-TERT1 | Two-part silicone elastomer; Pluronic and thrombin. | [47] |
| Vessel-like structures-on-a-chip | Coaxial nozzle-assisted extrusion-based bioprinting |  Reprinted with permission from ref. [48]. Copyright 2017 American Chemical Society | L929 fibroblasts; endothelial cells and smooth muscle cells | Cell-laden alginate filaments | [48] |
| Vessel-on-a-chip | - |  | HAECs; HASMC and NIH/3 T3 fibroblast cell lines | GelMA | [49] |
| Heart-on-a-Chip | Direct write bioprinter with a customized coaxial nozzle |  Reprinted with permission from ref. [50]. Copyright 2016 Elsevier | HUVECs | Alginate-GelMA | [50] |
| Myocardium-on-a-chip | Extrusion-based 3D bioprinting |  Reprinted with permission from ref. [51]. Copyright 2020 John Wiley and Sons. | hiPSC-CSs | Non-mulberry silk-based ink GelMA and PEGDMA | [51] |
| Gut-on-a-chip | Dual cell-printing system supplemented with a core-shell nozzle |  Reprinted with permission from ref. [52]. Copyright 2018 American Chemical Society. | Caco-2 cells and HUVECs | Cell-laden collagen bioinks | [52] |
| Thrombosis-on-a-chip | Embedded extrusion bioprinting |  Reprinted with permission from ref. [53]. Copyright 2016 Royal Society of Chemistry. | HUVECs | GelMA | [53] |
| Tumor array-on-a-chip | On-demand array printing |  | MDA-MB-231 breast tumor cells showed | GelMA | [54] |
| Placenta-on-a-chip | Extrusion-based 3D bioprinting |  Reprinted with permission from ref. [55]. Copyright 2016 American Chemical Society. | Human placental cell line and hMSCs | GelMA | [55] |
| 3D (Bio)Printing Technology | Schematic Representation | Ref. |
|---|---|---|
| Embedded extrusion bioprinting |  Reprinted from ref. [61]. | [61] |
| Embedded extrusion bioprinting |  | [62] |
| Embedded extrusion bioprinting |  Reprinted with permission from ref. [60]. Copyright 2019 Elsevier. | [60] |
| DLW and DLIP |  Reprinted from ref. [63]. | [63] |
| LIFT printing |  Reprinted with permission from ref. [64]. Copyright 2019 Royal Society of Chemistry. | [64] |
| SLA and Bioprinting (BioScaffolder) |  | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, V.; Gonçalves, I.; Lage, T.; Rodrigues, R.O.; Minas, G.; Teixeira, S.F.C.F.; Moita, A.S.; Hori, T.; Kaji, H.; Lima, R.A. 3D Printing Techniques and Their Applications to Organ-on-a-Chip Platforms: A Systematic Review. Sensors 2021, 21, 3304. https://doi.org/10.3390/s21093304
Carvalho V, Gonçalves I, Lage T, Rodrigues RO, Minas G, Teixeira SFCF, Moita AS, Hori T, Kaji H, Lima RA. 3D Printing Techniques and Their Applications to Organ-on-a-Chip Platforms: A Systematic Review. Sensors. 2021; 21(9):3304. https://doi.org/10.3390/s21093304
Chicago/Turabian StyleCarvalho, Violeta, Inês Gonçalves, Teresa Lage, Raquel O. Rodrigues, Graça Minas, Senhorinha F. C. F. Teixeira, Ana S. Moita, Takeshi Hori, Hirokazu Kaji, and Rui A. Lima. 2021. "3D Printing Techniques and Their Applications to Organ-on-a-Chip Platforms: A Systematic Review" Sensors 21, no. 9: 3304. https://doi.org/10.3390/s21093304