Magnetic Imprinted Polymer-Based Quartz Crystal Microbalance Sensor for Sensitive Label-Free Detection of Methylene Blue in Groundwater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
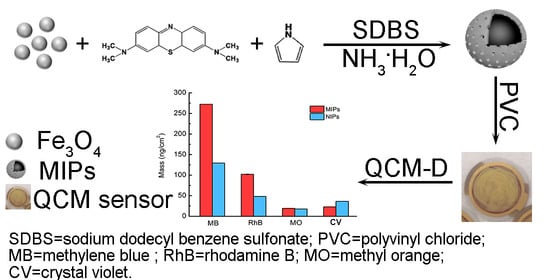
2.3. Preparation of MIPs
2.4. Binding Experiments
2.5. Immobilization of MIPs on QCM-D Sensors
2.6. Preparation of Water Samples
2.7. QCM-D Measurements
3. Results and Discussion
3.1. Preparation and Characterization of the MIPs-Coated QCM-D Sensors
3.2. Selecting the Surfactant Used in the MIPs-Coated QCM-D Sensors
3.3. Analytical Performance of the MIPs-Coated QCM-D Sensor
3.4. Selectivity and Reproducibility of the MIPs-Coated QCM-D Sensor
3.5. Application of the MIPs-Coated QCM-D Sensor
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qiao, X.X.; Zhang, X.J.; Tian, Y.; Meng, Y.G. Progresses on the theory and application of quartz crystal microbalance. Appl. Phys. Rev. 2016, 3, 031106. [Google Scholar] [CrossRef]
- Mujahid, A.; Afzal, A.; Dickert, F.L. An overview of high frequency acoustic sensors—QCMs, SAWs and FBARs—chemical and biochemical applications. Sensors 2019, 19, 4395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.Y.; Penn, L.S.; Xi, J. Quartz crystal microbalance: Sensing cell-substrate adhesion and beyond. Biosens. Bioelectron. 2017, 99, 593–602. [Google Scholar] [CrossRef]
- Kuchmenko, T.A.; Lvova, L.B. A perspective on recent advances in piezoelectric chemical sensors for environmental monitoring and foodstuffs analysis. Chemosensors 2019, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Emir Diltemiz, S.; Keçili, R.; Ersöz, A.; Say, R. Molecular imprinting technology in quartz crystal microbalance (QCM) Sensors. Sensors 2017, 17, 454. [Google Scholar] [CrossRef] [PubMed]
- BelBruno, J.J. Molecularly imprinted polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.R.; Feng, T.Y.; Xu, J.; Xue, C.H. Recent advances of molecularly imprinted polymer-based sensors in the detection of food safety hazard factors. Biosens. Bioelectron. 2019, 141, 111447. [Google Scholar] [CrossRef]
- Mujahid, A.; Mustafa, G.; Dickert, F.L. Label-free bioanalyte detection from nanometer to micrometer dimensions—molecular imprinting and QCMs. Biosensors 2018, 8, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battal, D.; Akgonullu, S.; Yalcin, M.S.; Yavuz, H.; Denizli, A. Molecularly imprinted polymer based quartz crystal microbalance sensor system for sensitive and label-free detection of synthetic cannabinoids in urine. Biosens. Bioelectron. 2018, 111, 10–17. [Google Scholar] [CrossRef]
- Pan, M.F.; Gu, Y.; Zhang, M.Y.; Wang, J.P.; Yun, Y.G.; Wang, S. Reproducible molecularly imprinted QCM sensor for accurate, stable, and sensitive detection of enrofloxacin residue in animal-derived food. Food Anal. Method. 2018, 11, 495–503. [Google Scholar] [CrossRef]
- Hu, Y.F.; Wang, C.; Li, X.D.; Liu, L.F. Preparation and application of epitope magnetic molecularly imprinted polymers for enrichment of sulfonamide antibiotics in water. Electrophoresis 2017, 38, 2462–2467. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.T.; Hu, X.L.; Liu, H.L.; Wang, Q.; He, C.Y. Rapid degradation of Congo red by molecularly imprinted polypyrrole-coated magnetic TiO2 nanoparticles in dark at ambient conditions. J. Hazard. Mater. 2015, 294, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Liu, J.; Xing, H.W.; Zhou, H.; Wu, M.H. Fabrication and application of magnetically catalytic imprinting nanozymes. ChemistrySelect 2020, 5, 8248–8288. [Google Scholar] [CrossRef]
- Fan, L.; Wei, C.; Xu, Q.; Xu, J. Polyppyrrole-coated cotton fabrics used for removal of methylene blue from aqueous solution. J. Textile Inst. 2017, 108, 1847–1852. [Google Scholar] [CrossRef]
- Badiee, H.; Zanjanchi, M.A.; Zamani, A.; Fashi, A. Hollow fiber liquid-phase microextraction based on the use of a rotating extraction cell: A green approach for trace determination of rhodamine 6G and methylene blue dyes. Environ. Pollut. 2019, 255, 113287. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.T.; Wang, X.H.; Huang, Y.Q.; Lai, K.Q.; Fan, Y.X. Rapid detection of trace methylene blue and malachite green in four fish tissues by ultra-sensitive surface-enhanced Raman spectroscopy coated with gold nanorods. Food Chem. 2019, 106, 106720. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Tang, J.; Crittenden, J.C. Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue. Chem. Eng. J. 2018, 335, 110–119. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef]
- Ayad, M.M.; Amer, W.A.; Zaghlol, S.; Minisy, L.M.; Bober, P.; Stejskal, J. Polypyrrole-coated cotton textile as adsorbent of methylene blue dye. Chem. Pap. 2018, 72, 1605–1618. [Google Scholar] [CrossRef]
- Yan, X.S.; Zhang, X.D.; Liu, H.L.; Liu, Y.H.; Ding, J.; Liu, Y.S.; Cai, Q.; Zhang, J.M. Fabrication of SDBS intercalated-reduced graphene oxide/polypyrrole nanocomposites for supercapacitors. Synthetic. Met. 2014, 196, 1–7. [Google Scholar] [CrossRef]
- Chen, J.; Feng, J.; Yan, W. Influence of metal oxides on the adsorption characteristics of ppy/metal oxides for methylene blue. J. Colloid Interf. Sci. 2016, 475, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Rico-Yuste, A.; Carrasco, S. Molecularly imprinted polymer-based hybrid materials for the development of optical sensors. Polymers 2019, 11, 1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reviakine, I.; Johannsmann, D.; Richter, R.P. Hearing what you cannot see and visualizing what you hear. Anal. Chem. 2011, 83, 8838–8848. [Google Scholar] [CrossRef] [PubMed]
- Borwitzky, H.; Haefeli, W.E.; Burhenne, J. Analysis of methylene blue in human urine by capillary electrophoresis. J. Chromatogr. B 2005, 826, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, X.M.; Wei, C.J.; Qu, Y.M.; Xiao, X.C.; Cheng, H. One-step synthesis of red-emitting carbon dots via a solvothermal method and its application in the detection of methylene blue. RSC Adv. 2019, 9, 29533–29540. [Google Scholar] [CrossRef] [Green Version]
- Zou, W.; Gong, L.; Huang, J.; Zhang, Z.; Zeng, H. Adsorption of hydrophobically modified polyacrylamide p(am-naaa-c16dmaac) on model coal and clay surfaces and the effect on selective flocculation of fine coal. Miner. Eng. 2019, 142, 105887. [Google Scholar] [CrossRef]
- Wang, L.Z.; Xu, Y.; Tan, X.J.; Tapas, S.; Zhang, J.L. Aim and shoot: Molecule-imprinting polymer coated MoO3 for selective SERS detection and photocatalytic destruction of low-level organic contaminants. RSC Adv. 2017, 7, 36201–36207. [Google Scholar] [CrossRef] [Green Version]
- Patskovsky, S.; Dallaire, A.; Meunier, M. Electrochemical surface plasmon resonance sensing with absorptive redox mediator film. Sens. Actuators B 2016, 222, 71–77. [Google Scholar] [CrossRef]









| Materials | Qe (mg·g−1) | Pseudo-Second-Order Model | ||
|---|---|---|---|---|
| k2 (g·mg−1·min−1) | Qeq (mg·g−1) | R2 | ||
| MIPs | 12.9 | 0.169 | 12.9 | 0.9998 |
| NIPs | 5.6 | 0.254 | 5.4 | 0.9904 |
| MIPs-SDS | 8.4 | 1.157 | 8.4 | 0.9999 |
| NIPs-SDS | 5.5 | 0.974 | 5.5 | 0.9999 |
| MIPs-PSS | 11.0 | 0.894 | 10.1 | 0.9994 |
| NIPs-PSS | 6.8 | 0.401 | 6.7 | 0.9975 |
| MIPs-PVP | 6.7 | 0.123 | 6.7 | 0.9995 |
| NIPs-PVP | 4.5 | 0.796 | 4.5 | 0.9991 |
| Samples | Matrix Calibration Curve | Matrix Effect |
|---|---|---|
| River water | y=1.6157x + 63.473 | 115% |
| Seawater | y=1.5590x + 48.788 | 128% |
| Samples | Cadded (µg/L) | Cfound (µg/L) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|
| River water | 50 | 48.4 | 96.8 | 4.2 |
| 100 | 95.6 | 96.4 | 4.1 | |
| 150 | 140.4 | 93.6 | 4.1 | |
| Seawater | 50 | 46.6 | 93.3 | 0.9 |
| 100 | 92.5 | 92.5 | 2.8 | |
| 150 | 129.5 | 86.3 | 1.3 |
| Methods | Linear Range (µg/L) | LOD (µg/L) | Reference |
|---|---|---|---|
| CE | 1.0 × 103–1.2 × 106 | 1.0 × 103 | [24] |
| UV-Vis spectrophotometry | 2.0–6.0 × 102 | 0.6 | [15] |
| HPLC-UV/Vis | 1.6–6.0 × 102 | 0.5 | [15] |
| Fluorescence | 16–3.0 × 103 | 3.2 | [25] |
| SERS | 3.2–3.2 × 108 | 3.2 | [26] |
| MIPs-SERS | - | 3.7 × 102 | [27] |
| SPR | 1.6 × 103–1.6 × 105 | 1.6 × 103 | [28] |
| QCM-D | 25–1.5 × 102 | 1.4 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Xing, H.; Li, G.; Wu, M. Magnetic Imprinted Polymer-Based Quartz Crystal Microbalance Sensor for Sensitive Label-Free Detection of Methylene Blue in Groundwater. Sensors 2020, 20, 5506. https://doi.org/10.3390/s20195506
Hu Y, Xing H, Li G, Wu M. Magnetic Imprinted Polymer-Based Quartz Crystal Microbalance Sensor for Sensitive Label-Free Detection of Methylene Blue in Groundwater. Sensors. 2020; 20(19):5506. https://doi.org/10.3390/s20195506
Chicago/Turabian StyleHu, Yufeng, Hanwen Xing, Gang Li, and Minghuo Wu. 2020. "Magnetic Imprinted Polymer-Based Quartz Crystal Microbalance Sensor for Sensitive Label-Free Detection of Methylene Blue in Groundwater" Sensors 20, no. 19: 5506. https://doi.org/10.3390/s20195506