N-Doped Reduced Graphene Oxide/Gold Nanoparticles Composite as an Improved Sensing Platform for Simultaneous Detection of Dopamine, Ascorbic Acid, and Uric Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Graphene-Based Materials
2.2. Preparation of Nanocomposites by AuNPs Electrodeposition
2.3. Characterization of the Electrochemical Performance of the Sensors
3. Results and Discussion
3.1. Morphological and Structural Characterization of the Materials
3.2. Electrochemical Sensing
3.2.1. Preliminary Evaluation of the Electrochemical Performance of the Electrodes Towards DA, AA, and UA Detection
3.2.2. Optimization of the Operational pH
3.2.3. Investigation of the Influence of the Scan Rate on Kinetics of DA Detection
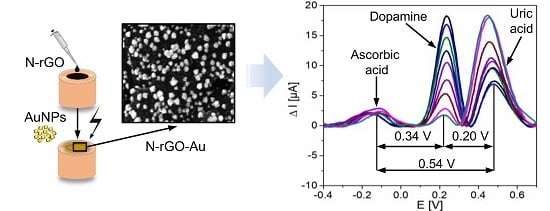
3.2.4. Simultaneous Determination of DA, AA, and UA
3.2.5. Reproducibility, Stability, and Selectivity of the GCE/N-rGO-Au Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jackowska, K.; Krysinski, P. New trends in the electrochemical sensing of dopamine. Anal. Bioanal. Chem. 2013, 405, 3753–3771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Du, J.; Wang, H.; Zou, C.; Jiang, F.; Yang, P.; Du, Y. A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2014, 204, 302–309. [Google Scholar] [CrossRef]
- Aydoğdu Tığ, G.; Günendi, G.; Pekyardımcı, Ş. A selective sensor based on Au nanoparticles-graphene oxide-poly(2,6-pyridinedicarboxylic acid) composite for simultaneous electrochemical determination of ascorbic acid, dopamine, and uric acid. J. Appl. Electrochem. 2017, 47, 607–618. [Google Scholar] [CrossRef]
- Si, B.; Song, E. Recent advances in the detection of neurotransmitters. Chemosensors 2018, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Preparation of Cu2O-reduced graphene nanocomposite modified electrodes towards ultrasensitive dopamine detection. Sensors 2018, 18, 199. [Google Scholar] [CrossRef] [Green Version]
- Sajid, M.; Nazal, M.K.; Mansha, M.; Alsharaa, A.; Jillani, S.M.S.; Basheer, C. Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid: A review. TrAC—Trends Anal. Chem. 2016, 76, 15–29. [Google Scholar] [CrossRef]
- Yang, L.; Liu, D.; Huang, J.; You, T. Simultaneous determination of dopamine, ascorbic acid and uric acid at electrochemically reduced graphene oxide modified electrode. Sens. Actuators B Chem. 2014, 193, 166–172. [Google Scholar] [CrossRef]
- Choo, S.S.; Kang, E.S.; Song, I.; Lee, D.; Choi, J.W.; Kim, T.H. Electrochemical detection of dopamine using 3D porous graphene oxide/gold nanoparticle composites. Sensors 2017, 17, 861. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef] [Green Version]
- Yi, S.Y.; Lee, J.H.; Hong, H.G. A selective determination of levodopa in the presence of ascorbic acid and uric acid using a glassy carbon electrode modified with reduced graphene oxide. J. Appl. Electrochem. 2014, 44, 589–597. [Google Scholar] [CrossRef]
- Chen, D.; Tang, L.; Li, J. Graphene-based materials in electrochemistry. Chem. Soc. Rev. 2010, 39, 3157–3180. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, Q.; Ai, L.; Tang, L.; Zhong, Y.; Loh, K.P. Hydrothermal dehydration for the “Green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties. Chem. Mater. 2009, 21, 2950–2956. [Google Scholar] [CrossRef]
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yu, Q.; Fu, W.; Chen, X.; Zhang, Q.; Dong, S.; Chen, H.; Zhang, S. A highly sensitive amperometric glutamate oxidase microbiosensor based on a reduced graphene oxide/prussian blue nanocube/gold nanoparticle composite film-modified pt electrode. Sensors 2020, 20, 2924. [Google Scholar] [CrossRef]
- Krishnendu, S.; Sarit, S.A.; Chaekyu, K.; Xiaoning, L.; Vincent, M.R. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2014, 112, 2739–2779. [Google Scholar]
- Guo, S.; Wang, E. Synthesis and electrochemical applications of gold nanoparticles. Anal. Chim. Acta 2007, 598, 181–192. [Google Scholar] [CrossRef]
- Díez, N.; Śliwak, A.; Gryglewicz, S.; Grzyb, B.; Gryglewicz, G. Enhanced reduction of graphene oxide by high-pressure hydrothermal treatment. RSC Adv. 2015, 5, 81831–81837. [Google Scholar] [CrossRef]
- Coros, M.; Varodi, C.; Pogacean, F.; Gal, E.; Pruneanu, S.M. Nitrogen-doped graphene: The influence of doping level on the charge-transfer resistance and apparent heterogeneous electron transfer rate. Sensors 2020, 20, 1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzyb, B.; Gryglewicz, S.; Śliwak, A.; Díez, N.; Machnikowski, J.; Gryglewicz, G. Guanidine, amitrole and imidazole as nitrogen dopants for the synthesis of N-graphenes. RSC Adv. 2016, 6, 15782–15787. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Qu, C.; Wang, G.; Wang, D. Simple synthesis of ZnO nanoparticles on N-doped reduced graphene oxide for the electrocatalytic sensing of l-cysteine. RSC Adv. 2017, 7, 35004–35011. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Z.H.; Zheng, X.Q.; Xu, J.Y.; Bao, W.J.; Wang, F.B.; Xia, X.H. Electrochemical sensor based on nitrogen doped graphene: Simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2012, 34, 125–131. [Google Scholar] [CrossRef]
- Wiench, P.; González, Z.; Menéndez, R.; Grzyb, B.; Gryglewicz, G. Beneficial impact of oxygen on the electrochemical performance of dopamine sensors based on N-doped reduced graphene oxides. Sens. Actuators B Chem. 2018, 257, 143–153. [Google Scholar] [CrossRef]
- Thearle, R.A.; Latiff, N.M.; Sofer, Z.; Mazánek, V.; Pumera, M. Boron and nitrogen doped graphene via microwave exfoliation for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Electroanalysis 2017, 29, 45–50. [Google Scholar] [CrossRef]
- Wiench, P.; Grzyb, B.; González, Z.; Menéndez, R.; Handke, B.; Gryglewicz, G. pH robust electrochemical detection of 4-nitrophenol on a reduced graphene oxide modified glassy carbon electrode. J. Electroanal. Chem. 2017, 787, 80–87. [Google Scholar] [CrossRef]
- Zhang, H.; Kuila, T.; Kim, N.H.; Yu, D.S.; Lee, J.H. Simultaneous reduction, exfoliation, and nitrogen doping of graphene oxide via a hydrothermal reaction for energy storage electrode materials. Carbon 2014, 69, 66–78. [Google Scholar] [CrossRef]
- Thanh, T.D.; Balamurugan, J.; Lee, S.H.; Kim, N.H.; Lee, J.H. Effective seed-assisted synthesis of gold nanoparticles anchored nitrogen-doped graphene for electrochemical detection of glucose and dopamine. Biosens. Bioelectron. 2016, 81, 259–267. [Google Scholar] [CrossRef]
- Maruyama, T.; Fujimoto, Y.; Maekawa, T. Synthesis of gold nanoparticles using various amino acids. J. Colloid Interface Sci. 2014, 447, 254–257. [Google Scholar] [CrossRef]
- Newman, J.D.S.; Blanchard, G.J. Formation of gold nanoparticles using amine reducing agents. Langmuir 2006, 22, 5882–5887. [Google Scholar] [CrossRef] [PubMed]
- Wiench, P.; González, Z.; Gryglewicz, S.; Menéndez, R.; Gryglewicz, G. Enhanced performance of pyrrolic N-doped reduced graphene oxide-modified glassy carbon electrodes for dopamine sensing. J. Electroanal. Chem. 2019, 852, 113547–113555. [Google Scholar] [CrossRef]
- Hnatejko, Z. Complexes of d- and f- metal ions with pyridine n-oxide and its derivates: Spectroscopic studies. Wiad. Chem. 2011, 65, 5–6. [Google Scholar]
- Muñoz, P.; Huenchuguala, S.; Paris, I.; Segura-Aguilar, J. Dopamine oxidation and autophagy. Parkinsons. Dis. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Sundar, S.; Venkatachalam, G.; Kwon, S.J. Biosynthesis of copper oxide (CuO) nanowires and their use for the electrochemical sensing of dopamine. Nanomaterials 2018, 8, 823. [Google Scholar] [CrossRef] [Green Version]
- Dorraji, P.S.; Jalali, F. Novel sensitive electrochemical sensor for simultaneous determination of epinephrine and uric acid by using a nanocomposite of MWCNTs-chitosan and gold nanoparticles attached to thioglycolic acid. Sens. Actuators B Chem. 2014, 200, 251–258. [Google Scholar] [CrossRef]
- Ping, J.; Wu, J.; Wang, Y.; Ying, Y. Simultaneous determination of ascorbic acid, dopamine and uric acid using high-performance screen-printed graphene electrode. Biosens. Bioelectron. 2012, 34, 70–76. [Google Scholar] [CrossRef]






| Sample | C | N | O | Au |
|---|---|---|---|---|
| rGO | 83.3 | - | 16.7 | - |
| rGO-Au | 76.8 | - | 14.6 | 6.2 |
| N-rGO | 84.8 | 6.4 | 8.8 | - |
| N-rGO-Au | 80.7 | 2.7 | 9.0 | 7.6 |
| Sample | C1s Peak Deconvolution | N1s Peak Deconvolution | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Csp2 | C-O/C-N | C=O | O=C-OH | N6 | NC | N5 | NQ | NX | |
| rGO | 54.1 | 18.0 | 5.7 | 5.1 | - | - | - | - | - |
| rGO-Au | 40.0 | 22.3 | 8.5 | 5.9 | - | - | - | - | - |
| N-rGO | 50.3 | 24.8 | 7.7 | 2.0 | 2.6 | 0.5 | 1.9 | 0.9 | 0.5 |
| N-rGO-Au | 48.9 | 26.3 | 4.6 | 0.9 | 0.7 | 1.0 | 0.7 | 0.3 | - |
| Electrodes | LOD [µM] | Linear Range [µM] | Sensitivity [µA µM-1] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DA | AA | UA | DA | AA | UA | DA | AA | UA | |
| GCE/rGO-Au | 3.9 | 57 | 68 | 8–80 | 100–1500 | 250–1500 | 0.41 | 0.003 | 0.003 |
| GCE/N-rGO-Au | 2.4 | 58 | 8.7 | 3–100 | 550–1500 | 20–1000 | 0.19 | 0.002 | 0.034 |
| Analyte | Added | Measured | Detection Performance | RSD |
|---|---|---|---|---|
| [µM] | [µM] | [%] | [%] | |
| DA | 50 | 50.7 | 101.4 | 3.5 |
| 46.8 | 93.6 | |||
| 47.5 | 95.0 | |||
| AA | 600 | 485.0 | 80.8 | 5.2 |
| 505.0 | 84.2 | |||
| 445.0 | 74.2 | |||
| UA | 100 | 82.4 | 82.4 | 1.6 |
| 79.7 | 79.7 | |||
| 79.4 | 79.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minta, D.; González, Z.; Wiench, P.; Gryglewicz, S.; Gryglewicz, G. N-Doped Reduced Graphene Oxide/Gold Nanoparticles Composite as an Improved Sensing Platform for Simultaneous Detection of Dopamine, Ascorbic Acid, and Uric Acid. Sensors 2020, 20, 4427. https://doi.org/10.3390/s20164427
Minta D, González Z, Wiench P, Gryglewicz S, Gryglewicz G. N-Doped Reduced Graphene Oxide/Gold Nanoparticles Composite as an Improved Sensing Platform for Simultaneous Detection of Dopamine, Ascorbic Acid, and Uric Acid. Sensors. 2020; 20(16):4427. https://doi.org/10.3390/s20164427
Chicago/Turabian StyleMinta, Daria, Zoraida González, Piotr Wiench, Stanisław Gryglewicz, and Grażyna Gryglewicz. 2020. "N-Doped Reduced Graphene Oxide/Gold Nanoparticles Composite as an Improved Sensing Platform for Simultaneous Detection of Dopamine, Ascorbic Acid, and Uric Acid" Sensors 20, no. 16: 4427. https://doi.org/10.3390/s20164427