Probing the Cyanobacterial Microcystis Gas Vesicles after Static Pressure Treatment: A Potential In Situ Rapid Method
Abstract
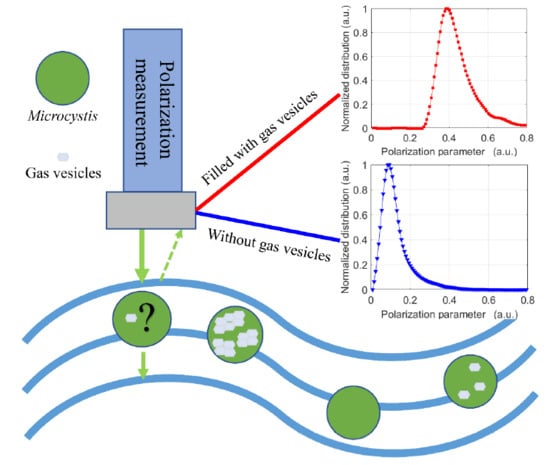
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Experimental Setup for Polarization Measurement
2.3. Analytical Methods
2.4. The Pressurization Device
3. Results
3.1. Effectiveness of Polarized Light Scattering Method on Monitoring the Collapse of Gas Vesicles of Microcystis Cells after Static Pressure Treatments
3.1.1. The Distributions of the Polarization Parameter and R-Value of Microcystis Cells after Static Pressure Treatments
3.1.2. TEM Images of WT after Different Static Pressure Treatments
3.1.3. Settlement Performances of WT after Static Pressure Treatments
3.2. Effectiveness of Polarized Light Scattering Method on Monitoring Regeneration of Gas Vesicles of Microcystis Cells during Post-Pressurization Incubation
3.2.1. The Distributions of Polarization Parameter and R-Value of Microcystis Cells during Post-Pressurization Incubation
3.2.2. TEM Images of WT during Post-Pressurization Incubation
3.2.3. Settlement Performances of WT during Post-Pressurization Incubation
4. Discussion
4.1. The Changes of Turbidity and the Relationship between R and Turbidity
4.2. Comparison of Light Intensity and Polarization Part
4.3. The Micrograph of WT Sample
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Boegehold, A.G.; Johnson, N.S.; Kashian, D.R. Dreissenid (quagga and zebra mussel) veligers are adversely affected by bloom forming cyanobacteria. Ecotoxicol. Environ. Saf. 2019, 182, 109426. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Genet. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Schaefer, A.M.; Yrastorza, L.; Stockley, N.; Harvey, K.; Harris, N.; Grady, R.; Sullivan, J.; McFarland, M.; Reif, J.S. Exposure to microcystin among coastal residents during a cyanobacteria bloom in Florida. Harmful Algae 2020, 92, 101769. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Xu, H.; McCarthy, M.J.; Zhu, G.; Qin, B.; Li, Y.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Huang, Y.; Hu, J.; Li, P.; Zhang, C.; Li, L.; Xu, P.; Zhang, J.; Chen, X. The nitrogen reduction in eutrophic water column driven by Microcystis blooms. J. Hazard. Mater. 2020, 385, 121578. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.; Cho, K.; Lee, H.; Kang, T.; Kim, J.H. The relative importance of water temperature and residence time in predicting cyanobacteria abundance in regulated rivers. Water Res. 2017, 124, 11–19. [Google Scholar] [CrossRef]
- Griffith, A.W.; Gobler, C.J. Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 2019, 91, 101590. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nakahara, H. The formation and degradation of cyanobacterium Aphanizomenon flos-aquae blooms: The importance of pH, water temperature, and day length. Limnology 2005, 6, 1–6. [Google Scholar] [CrossRef]
- Oliver, R.; Walsby, A. Buoyancy and suspension of planktonic cyanobacteria. Methods Enzymol. 1988, 167, 521–527. [Google Scholar] [CrossRef]
- Clark, A.E.; Walsby, A.E. The development and vertical distribution of populations of gas-vacuolate bacteria in a eutrophic, monomictic lake. Arch. Microbiol. 1978, 118, 229–233. [Google Scholar] [CrossRef]
- Paerl, H.W. A comparison of cyanobacterial bloom dynamics in freshwater, estuarine and marine environments. Phycologia 1996, 35, 25–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Ryan, J.P.; Kieft, B.; Hobson, B.W.; McEwen, R.S.; Godin, M.A.; Harvey, J.B.; Barone, B.; Bellingham, J.G.; Birch, J.M.; et al. Targeted Sampling by Autonomous Underwater Vehicles. Front. Mar. Sci. 2019, 6, 415. [Google Scholar] [CrossRef] [Green Version]
- Gaget, V.; Hobson, P.; Keulen, A.; Newton, K.; Monis, P.; Humpage, A.R.; Weyrich, L.S.; Brookes, J.D. Toolbox for the sampling and monitoring of benthic cyanobacteria. Water Res. 2020, 169, 115222. [Google Scholar] [CrossRef]
- Bertone, E.; Burford, M.A.; Hamilton, D.P. Fluorescence probes for real-time remote cyanobacteria monitoring: A review of challenges and opportunities. Water Res. 2018, 141, 152–162. [Google Scholar] [CrossRef]
- Greenstein, K.E.; Wert, E.C. Using rapid quantification of adenosine triphosphate (ATP) as an indicator for early detection and treatment of cyanobacterial blooms. Water Res. 2019, 154, 171–179. [Google Scholar] [CrossRef]
- Cannizzaro, J.P.; Barnes, B.B.; Hu, C.; Corcoran, A.A.; Hubbard, K.A.; Muhlbach, E.; Sharp, W.C.; Brand, L.E.; Kelble, C.R. Remote detection of cyanobacteria blooms in an optically shallow subtropical lagoonal estuary using MODIS data. Remote Sens. Environ. 2019, 231, 111227. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, N.; Yang, S.; Ren, H.; Ge, Y.; Feng, P.; Dong, B.; Zhao, Y. Predicting cyanobacteria bloom occurrence in lakes and reservoirs before blooms occur. Sci. Total Environ. 2019, 670, 837–848. [Google Scholar] [CrossRef]
- Walsby, A.E. Gas vesicles. Microbiol. Mol. Biol. Rev. 1994, 58, 94–144. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Walsby, A.E. Water-blooms. Biol. Rev. 1975, 50, 437–481. [Google Scholar] [CrossRef]
- Lehmann, H.; Jost, M. Kinetics of the assembly of gas vacuoles in the blue-green alga Microcystis aeruginosa Kuetz. emend. Elekin. Arch. Microbiol. 1971, 79, 59–68. [Google Scholar] [CrossRef]
- Walsby, A.; Kinsman, R.; George, K. The measurement of gas vesicle volume and buoyant density in planktonic bacteria. J. Microbiol. Methods 1992, 15, 293–309. [Google Scholar] [CrossRef]
- Porat, R.; Teltsch, B.; Mosse, R.; Dubinsky, Z.; Walsby, A. Turbidity changes caused by collapse of cyanobacterial gas vesicles in water pumped from lake Kinneret into the Israeli national water carrier. Water Res. 1999, 33, 1634–1644. [Google Scholar] [CrossRef]
- Lee, T.J.; Nakano, K.; Matsumura, M. A new method for the rapid evaluation of gas vacuoles regeneration and viability of cyanobacteria by flow cytometry. Biotechnol. Lett. 2000, 22, 1833–1838. [Google Scholar] [CrossRef]
- Dyer, S.W.; Needoba, J.A. Use of High-Resolution Pressure Nephelometry To Measure Gas Vesicle Collapse as a Means of Determining Growth and Turgor Changes in Planktonic Cyanobacteria. Appl. Environ. Microbiol. 2020, 86, 01790-19. [Google Scholar] [CrossRef] [PubMed]
- Walsby, A.E. The pressure relationships of gas vacuoles. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1971, 178, 301–326. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.; Li, P.; Chen, G.; Peng, L.; Pan, L. Pressurized Microcystis can help to remove nitrate from eutrophic water. Bioresour. Technol. 2018, 248, 140–145. [Google Scholar] [CrossRef]
- Dubelaar, G.B.J.; Visser, J.W.M.; Donze, M. Anomalous behaviour of forward and perpendicular light scattering of a cyanobacterium owing to intracellular gas vacuoles. Cytometry 1987, 8, 405–412. [Google Scholar] [CrossRef]
- Matthews, M.W.; Bernard, S. Using a two-layered sphere model to investigate the impact of gas vacuoles on the inherent optical properties of Microcystis aeruginosa. Biogeosciences 2013, 10, 8139–8157. [Google Scholar] [CrossRef] [Green Version]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley and Sons: New York, NY, USA, 1983. [Google Scholar] [CrossRef]
- Sun, M.; He, H.; Zeng, N.; Du, E.; Guo, Y.; Liu, S.; Wu, J.; He, Y.; Ma, H. Characterizing the microstructures of biological tissues using Mueller matrix and transformed polarization parameters. Biomed. Opt. Express 2014, 5, 4223–4234. [Google Scholar] [CrossRef] [Green Version]
- Chami, M. Importance of the polarization in the retrieval of oceanic constituents from the remote sensing reflectance. J. Geophys. Res. Space Phys. 2007, 112, 05026. [Google Scholar] [CrossRef]
- Li, X.; Liao, R.; Zhou, J.; Leung, P.T.; Yan, M.; Ma, H. Classification of morphologically similar algae and cyanobacteria using Mueller matrix imaging and convolutional neural networks. Appl. Opt. 2017, 56, 6520–6530. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, J.; Liao, R.; Zhou, J.; Meng, F.; Yao, Y.; Chen, H.; Tao, Y.; Ma, H. Characterization of physiological states of the suspended marine microalgae using polarized light scattering. Appl. Opt. 2020, 59, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Li, Q.; Mao, X. A prototype for detection of particles in sea water by using polarize-light scattering. In Proceedings of the OCEANS 2019, Marseille, France, 17–20 June 2019. [Google Scholar] [CrossRef]
- Rajasekhar, P.; Fan, L.-H.; Nguyen, T.; Roddick, F.A. A review of the use of sonication to control cyanobacterial blooms. Water Res. 2012, 46, 4319–4329. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liao, R.; Tao, Y.; Liu, Z.; Wang, Y.; Ma, H. Evaluation for gas vesicles of sonicated cyanobacteria using polarized light scattering. Optik 2020, 216, 164835. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, R.; Dai, J.; Liu, Z.; Xiong, Z.; Zhang, T.; Chen, H.; Ma, H. Differentiation of suspended particles by polarized light scattering at 120°. Opt. Express 2018, 26, 22419–22431. [Google Scholar] [CrossRef]
- Luo, S.; Chen, Z. A procedure of linear discrimination analysis with detected sparsity structure for high-dimensional multi-class classification. J. Multivar. Anal. 2020, 179, 104641. [Google Scholar] [CrossRef]
- Fisher, R.A. The use of multiple measurements in taxonomic problems. Ann. Eugen. 1936, 7, 179–188. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zeng, J.; Song, W.; Yu, X. Comparing the effects of chlorination on membrane integrity and toxin fate of high- and low-viability cyanobacteria. Water Res. 2020, 177, 115769. [Google Scholar] [CrossRef]
- Cong, H.; Sun, F.; Chen, W.; Xu, Y.; Wang, W. Study on the method and mechanism of pre-pressure coagulation and sedimentation for Microcystis removal from drinking-water sources. Environ. Technol. 2017, 39, 433–449. [Google Scholar] [CrossRef]
- Sun, D.; Li, Y.; Wang, Q.; Gao, J.; Lv, H.; Le, C.; Huang, C. Light scattering properties and their relation to the biogeochemical composition of turbid productive waters: A case study of Lake Taihu. Appl. Opt. 2009, 48, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Song, L.; Li, R. Taxonomic notes on water bloom forming Microcystis species (Cyanophyta) from China—An example from samples of the Dianchi Lake. Acta Phytotaxon. Sin. 2007, 45, 727. [Google Scholar] [CrossRef]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liao, R.; Tao, Y.; Zhuo, Z.; Liu, Z.; Deng, H.; Ma, H. Probing the Cyanobacterial Microcystis Gas Vesicles after Static Pressure Treatment: A Potential In Situ Rapid Method. Sensors 2020, 20, 4170. https://doi.org/10.3390/s20154170
Li J, Liao R, Tao Y, Zhuo Z, Liu Z, Deng H, Ma H. Probing the Cyanobacterial Microcystis Gas Vesicles after Static Pressure Treatment: A Potential In Situ Rapid Method. Sensors. 2020; 20(15):4170. https://doi.org/10.3390/s20154170
Chicago/Turabian StyleLi, Jiajin, Ran Liao, Yi Tao, Zepeng Zhuo, Zhidi Liu, Hanbo Deng, and Hui Ma. 2020. "Probing the Cyanobacterial Microcystis Gas Vesicles after Static Pressure Treatment: A Potential In Situ Rapid Method" Sensors 20, no. 15: 4170. https://doi.org/10.3390/s20154170