Growth Stages Classification of Potato Crop Based on Analysis of Spectral Response and Variables Optimization
Abstract
:1. Introduction
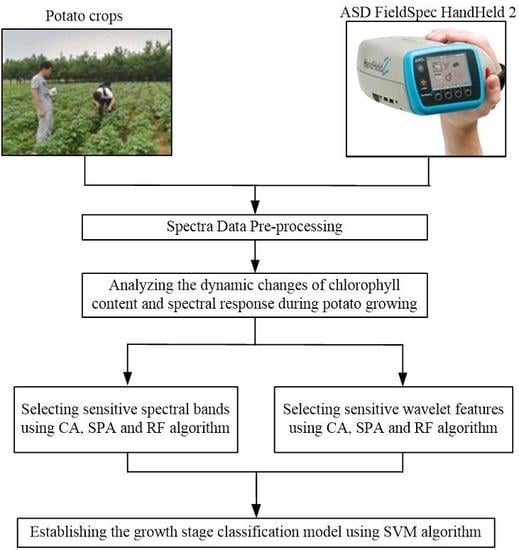
2. Materials and Methods
2.1. Spectral Data Collection
2.2. Chlorophyll Content Measurement
2.3. Pre-Processing of Spectral Data
2.4. Sample Subset Partitioning
2.5. Continuous Wavelet Transformation
2.6. Variables Selection Algorithm
2.6.1. Successive Projection Algorithm
2.6.2. Random Frog Algorithm
2.7. Support Vector Machine Modeling Method
3. Results
3.1. Statistics on Chlorophyll Content and Sample Set Partition
3.2. Analysis of Spectral Response During Growth
3.3. Correlation between Chlorophyll Content and Reflectance Spectra
3.4. Sensitive Wavelengths Selection for Dynamic Growth
3.4.1. Correlation Analysis between Growth Stages and Reflectance Spectra
3.4.2. Sensitive Wavelengths Selection Using SPA
3.4.3. Sensitive Wavelengths Selection Using RF
3.5. Sensitive Spectral Variables Selection Based Continuous Wavelet Analysis
3.5.1. Correlation Analysis between Growth Stages and Wavelet Features
3.5.2. Selection of Sensitive Wavelet Features Using SPA
3.5.3. Selection of Sensitive Wavelet Features Using RF
3.6. Establishing Growth Stage Identification Model Using SVM
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mavridou, E.; Vrochidou, E.; Papakostas, G.A.; Pachidis, T.; Kaburlasos, V.G. Machine vision systems in precision agriculture for crop farming. J. Imaging 2019, 5, 89. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.R.; Chao, K.; Kim, M.S. Machine vision technology for agricultural applications. Comput. Electron. Agric. 2002, 36, 173–191. [Google Scholar] [CrossRef] [Green Version]
- Günel, E.; Karadoĝan, T. Effect of irrigation applied at different growth stages and length of irrigation period on quality characters of potato tubers. Potato Res. 1998, 41, 9–19. [Google Scholar] [CrossRef]
- Shillito, R.M.; Timlin, D.; Fleisher, D.H.; Reddy, V.; Quebedeaux, B. Yield response of potato to spatially patterned nitrogen application. Agric. Ecosyst. Environ. 2009, 129, 107–116. [Google Scholar] [CrossRef]
- Awgchew, H.; Gebremedhin, H.; Taddesse, G.; Alemu, D. Influence of nitrogen rate on nitrogen use efficiency and quality of potato (Solanum tuberosum L.) varieties at Debre Berhan, central highlands of Ethiopia. Int. J. Soil Sci. 2016, 12, 10–17. [Google Scholar] [CrossRef]
- Zotarelli, L.; Rens, L.R.; Cantliffe, D.J.; Stoffella, P.J.; Gergela, D.; Burhans, D. Rate and timing of nitrogen fertilizer application on potato ‘FL1867’. Part I: Plant nitrogen uptake and soil nitrogen availability. Field Crop. Res. 2015, 183, 246–256. [Google Scholar] [CrossRef]
- Alva, A.K.; Fan, M.; Qing, C.; Rosen, C.; Ren, H. Improving Nutrient-Use efficiency in Chinese potato production: Experiences from the United States. J. Crop Improv. 2011, 25, 46–85. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Yang, G.; Haikuan, F. Variations in crop variables within wheat canopies and responses of canopy spectral characteristics and derived vegetation indices to different vertical leaf layers and spikes. Remote Sens. Environ. 2015, 169, 358–374. [Google Scholar] [CrossRef]
- Igamberdiev, R.M.; Bill, R.; Schubert, H.; Lennartz, B. Analysis of cross-seasonal spectral response from Kettle Holes: Application of remote sensing techniques for chlorophyll estimation. Remote Sens. 2012, 4, 3481–3500. [Google Scholar] [CrossRef] [Green Version]
- Wen, P.-F.; He, J.; Ning, F.; Wang, R.; Zhang, Y.-H.; Li, J. Estimating leaf nitrogen concentration considering unsynchronized maize growth stages with canopy hyperspectral technique. Ecol. Indic. 2019, 107, 1–16. [Google Scholar] [CrossRef]
- Hartmann, T.E.; Yue, S.; Schulz, R.; Chen, X.; Zhang, F.; Müller, T. Nitrogen dynamics, apparent mineralization and balance calculations in a maize—wheat double cropping system of the North China Plain. Field Crop. Res. 2014, 160, 22–30. [Google Scholar] [CrossRef]
- Wen, P.-F.; Wang, R.; Shi, Z.-J.; Ning, F.; Wang, S.-L.; Zhang, Y.-J.; Zhang, Y.-H.; Wang, Q.; Li, J. Effects of N application rate on n remobilization and accumulation in maize (zea mays, l.) and estimating of vegetative n remobilization using hyperspectral measurements. Comput. Electron. Agric. 2018, 152, 166–181. [Google Scholar] [CrossRef]
- Sun, H.; Zheng, T.; Liu, N.; Cheng, M.; Li, M.; Zhang, Q. Vertical distribution of chlorophyll in potato plants based on hyperspectral imaging. Trans. Chin. Soc. Agric. Eng. 2018, 34, 149–156. [Google Scholar] [CrossRef]
- Yu, K.; Lenz-Wiedemann, V.; Chen, X.; Bareth, G. Estimating leaf chlorophyll of barley at different growth stages using spectral indices to reduce soil background and canopy structure effects. ISPRS J. Photogramm. Remote Sens. 2014, 97, 58–77. [Google Scholar] [CrossRef]
- Graeff, S.; Claupein, W. Identification and discrimination of water stress in wheat leaves (Triticum aestivumL.) by means of reflectance measurements. Irrig. Sci. 2007, 26, 61–70. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum L. and Acer platanoides L. leaves. spectral features and relation to Chlorophyll Estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Ekaterina, S.; Vladimir, S. Connection of the Photochemical Reflectance Index (PRI) with the Photosystem II Quantum yield and nonphotochemical quenching can be dependent on variations of photosynthetic parameters among investigated plants: A meta-analysis. Remote Sens. 2018, 10, 771. [Google Scholar] [CrossRef] [Green Version]
- Penuelas, J.; Baret, F.; Filella, I. Semiempirical indexes to assess carotenoids Chlorophyll-a Ratio from leaf spectral reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar] [CrossRef]
- Peñuelas, J.; Pinol, J.; Ogaya, R.; Filella, I. Estimation of plant water concentration by the reflectance Water Index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, H.; Dai, X.; Qin, Q.; Li, J.; Zhang, T.; Sun, Y. Spectral characteristics of copper-stressed vegetation leaves and further understanding of the copper stress vegetation index. Int. J. Remote Sens. 2019, 40, 4473–4488. [Google Scholar] [CrossRef]
- Liu, N.; Wu, L.; Chen, L.; Sun, H.; Dong, Q.; Wu, J. Spectral characteristics analysis and water content detection of potato plants leaves. IFAC-PapersOnLine 2018, 51, 541–546. [Google Scholar] [CrossRef]
- Cerra, D.; Agapiou, A.; Cavalli, R.M.; Sarris, A. An objective assessment of hyperspectral indicators for the detection of buried archaeological relics. Remote Sens. 2018, 10, 500. [Google Scholar] [CrossRef]
- Cai, W.; Li, Y.; Shao, X. A variable selection method based on uninformative variable elimination for multivariate calibration of near-infrared spectra. Chemom. Intell. Lab. Syst. 2008, 90, 188–194. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, C.; Weng, H.; Nie, P.; He, Y. Sensitive wavelengths selection in Identification of Ophiopogon japonicus based on near-infrared hyperspectral imaging technology. Int. J. Anal. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Qiao, L.; Xing, Z.; Li, M.; Sun, H.; Zhang, J.; Zhang, Y. Detection of chlorophyll content in growth potato based on spectral variable analysis. Spectrosc Lett. 2020, 53, 476–488. [Google Scholar] [CrossRef]
- Mehmood, T.; Liland, K.H.; Snipen, L.; Sæbø, S. A review of variable selection methods in Partial Least Squares Regression. Chemom. Intell. Lab. Syst. 2012, 118, 62–69. [Google Scholar] [CrossRef]
- Li, H.D.; Xu, Q.S.; Liang, Y.Z. libPLS: An integrated library for partial least squares regression and linear discriminant analysis. Chemom. Intell. Lab. Syst. 2018, 176, 34–43. [Google Scholar] [CrossRef]
- Sun, H.; Liu, N.; Wu, L.; Zheng, T.; Li, M.; Wu, J. Visualization of water content distribution in potato leaves based on hyperspectral image. Spectrosc. Spectr. Anal. 2019, 39, 910–916. [Google Scholar] [CrossRef]
- Cheng, T.; Rivard, B.; Sánchez-Azofeifa, A. Spectroscopic determination of leaf water content using continuous wavelet analysis. Remote Sens. Environ. 2011, 115, 659–670. [Google Scholar] [CrossRef]
- Li, D.; Cheng, T.; Zhou, K.; Zheng, H.; Yao, X.; Tian, Y.; Zhu, Y.; Cao, W. WREP: A wavelet-based technique for extracting the red edge position from reflectance spectra for estimating leaf and canopy chlorophyll contents of cereal crops. ISPRS J. Photogramm. Remote Sens. 2017, 129, 103–117. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Zheng, H.; Zhou, K.; Yao, X.; Tian, Y.; Zhu, Y.; Cao, W.; Cheng, T. Estimation of area- and mass-based leaf nitrogen contents of wheat and rice crops from water-removed spectra using continuous wavelet analysis. Plant Methods 2018, 14, 76–96. [Google Scholar] [CrossRef]
- Wang, H.F.; Huo, Z.G.; Zhou, G.S.; Liao, Q.H.; Feng, H.K.; Wu, L. Estimating leaf SPAD values of freeze-damaged winter wheat using continuous wavelet analysis. Plant Physiol. Biochem. 2016, 98, 39–45. [Google Scholar] [CrossRef]
- Lu, J.-J.; Huang, W.-J.; Zhang, J.-C.; Jiang, J.-B. Quantitative identification of yellow rust and powdery mildew in winter wheat based on wavelet feature. Spectrosc. Spectr. Anal. 2016, 36, 1854–1858. [Google Scholar] [CrossRef]
- Clevers, J.G.; Kooistra, L. Using hyperspectral remote sensing data for retrieving canopy chlorophyll and nitrogen content. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 574–583. [Google Scholar] [CrossRef]
- Cohen, Y.; Alchanatis, V.; Zusman, Y.; Dar, Z.; Bonfil, D.J.; Karnieli, A.; Zilberman, A.; Moulin, A.; Ostrovsky, V.; Levi, A.; et al. Leaf nitrogen estimation in potato based on spectral data and on simulated bands of the satellite. Precis. Agric. 2010, 11, 520–537. [Google Scholar] [CrossRef]
- Sun, H.; Liu, N.; Xing, Z.; Zhang, Z.; Li, M.; Wu, J. Parameter optimization of potato spectral response characteristics and growth stage identification. Spectrosc. Spectr. Anal. 2019, 39, 1870–1877. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Haitao, S.; Peiqiang, Y. Comparison of grating-based near-infrared (NIR) and Fourier transform mid-infrared (ATR-FT/MIR) spectroscopy based on spectral preprocessing and wavelength selection for the determination of crude protein and moisture content in wheat. Food Control 2017, 82, 57–65. [Google Scholar] [CrossRef]
- Fearn, T.; Riccioli, C.; Garrido-Varo, A.; Guerrero-Ginel, J.E. On the geometry of SNV and MSC. Chemom. Intell. Lab. Syst. 2009, 96, 22–26. [Google Scholar] [CrossRef]
- Galvão, R.K.H.; De Araújo, M.C.U.; José, G.E.; Pontes, M.J.; Silva, E.C.; Saldanha, T.C.B. A method for calibration and validation subset partitioning. Talanta 2005, 67, 736–740. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, L.; Li, M.; Wang, Y.; Sheng, D.; Liu, J.; Wang, C. Weighted SPXY method for calibration set selection for composition analysis based on near-infrared spectroscopy. Infrared Phys. Technol. 2018, 95, 88–92. [Google Scholar] [CrossRef]
- Li, H.D.; Liang, Y.Z.; Xu, Q.S.; Cao, D.S. Model population analysis for variable selection. J. Chemom. 2009, 24, 418–423. [Google Scholar] [CrossRef]
- Araújo, M.C.U.; Saldanha, T.C.B.; Galvão, R.K.H.; Yoneyama, T.; Chame, H.C.; Visani, V. The successive projections algorithm for variable selection in spectroscopic multicomponent analysis. Chemom. Intell. Lab. Syst. 2001, 57, 65–73. [Google Scholar] [CrossRef]
- Hu, F.; Zhou, M.; Yan, P.; Li, D.; Lai, W.; Zhu, S.; Wang, Y. Selection of characteristic wavelengths using SPA for laser induced fluorescence spectroscopy of mine water inrush. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 367–374. [Google Scholar] [CrossRef]
- Li, H.D.; Xu, Q.S.; Liang, Y.Z. Random Frog: An efficient reversible jump Markov Chain Monte Carlo-like approach for gene selection and disease classification. Anal. Chim. Acta 2012, 740, 20–26. [Google Scholar] [CrossRef]
- Yun, Y.-H.; Li, H.-D.; Wood, L.R.E.; Fan, W.; Wang, J.-J.; Cao, N.-S.; Xu, Q.-S.; Liang, Y. An efficient method of wavelength interval selection based on random frog for multivariate spectral calibration. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 111, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.H.; Lee, S.L. A multi-class SVM classification system based on learning methods from indistinguishable chinese official documents. Expert Syst. Appl. 2012, 39, 3127–3134. [Google Scholar] [CrossRef]
- Saberioon, M.M.; Amin, M.S.M.; Anuar, A.R.; Gholizadeh, A.; Wayayok, A.; Khairunniza-Bejo, S. Assessment of rice leaf chlorophyll content using visible bands at different growth stages at both the leaf and canopy scale. Int. J. Appl. Earth Obs. 2014, 32, 35–45. [Google Scholar] [CrossRef]
- Feng, W.; Guo, B.-B.; Wang, Z.-J.; He, L.; Song, X.; Wang, Y.-H.; Guo, T. Measuring leaf nitrogen concentration in winter wheat using double-peak spectral reflection remote sensing data. Field Crop. Res. 2014, 159, 43–52. [Google Scholar] [CrossRef]
- Quemada, M.; Daughtry, C.S.T. Spectral Indices to improve crop residue cover estimation under varying moisture conditions. Remote Sens. 2016, 8, 660. [Google Scholar] [CrossRef] [Green Version]
- Jingcheng, Z.; Ning, W.; Lin, Y.; Fengnong, C.; Kaihua, W. Discrimination of winter wheat disease and insect stresses using continuous wavelet features extracted from foliar spectral measurements. Biosyst. Eng. 2017, 162, 20–29. [Google Scholar] [CrossRef]
- Huang, S.; Miao, Y.; Yuan, F.; Cao, Q.; Ye, H.; Lenz-Wiedemann, V.I.; Bareth, G. In-Season diagnosis of rice nitrogen status using proximal fluorescence canopy sensor at different growth stages. Remote Sens. 2019, 11, 1847. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chen, J.; Fan, Y.; Cheng, Y.; Wu, X.; Zhang, J.; Wang, B.; Wang, X.; Yong, T.; Liu, W.; et al. Evaluating photosynthetic pigment contents of maize using UVE-PLS based on continuous wavelet transform. Comput. Electron. Agric. 2020, 169, 105–160. [Google Scholar] [CrossRef]
- Andries, J.P.M.; Vander Heyden, Y.; Buydens, L.M.C. Improved variable reduction in partial least squares modelling based on Predictive-Property-Ranked Variables and adaptation of partial least squares complexity. Anal. Chim. Acta 2011, 705, 292–305. [Google Scholar] [CrossRef]
- Li, D.; Cheng, T.; Jia, M.; Zhou, K.; Lu, N.; Yao, X.; Tian, Y.; Zhu, Y.; Cao, W. PROCWT: Coupling PROSPECT with continuous wavelet transform to improve the retrieval of foliar chemistry from leaf bidirectional reflectance spectra. Remote Sens. Environ. 2018, 206, 1–14. [Google Scholar] [CrossRef]
- Li, L.; Ren, T.; Ma, Y.; Wei, Q.; Wang, S.; Li, X.; Cong, R.-H.; Liu, S.; Lu, J. Evaluating chlorophyll density in winter oilseed rape (Brassica napus L.) using canopy hyperspectral red-edge parameters. Comput. Electron. Agric. 2016, 126, 21–31. [Google Scholar] [CrossRef]











| Growth Stage | Potato Crop Characteristics | Collection Date | Samples |
|---|---|---|---|
| S1 | Appearing flower buds, having about 12 leaves | 15 May | 74 |
| S2 | Appearing flowers | 24 May | 80 |
| S3 | Flowers falling, stems and leaves aging, and the lower leaves being yellow | 7 June | 80 |
| S4 | Stems and leaves withering, and the upper leaves being yellow | 19 June | 80 |
| Samples | Data Set | Sample Number | Max | Min | Mean | SD |
|---|---|---|---|---|---|---|
| S1 | all | 74 | 40.77 | 17.64 | 28.12 | 5.05 |
| train | 50 | 40.77 | 17.64 | 28.27 | 5.31 | |
| test | 24 | 33.12 | 19.64 | 27.48 | 3.86 | |
| S2 | all | 80 | 41.20 | 16.30 | 31.04 | 5.81 |
| train | 50 | 41.20 | 16.30 | 30.23 | 6.29 | |
| test | 30 | 37.46 | 25.26 | 33.45 | 3.04 | |
| S3 | all | 80 | 35.63 | 13.70 | 22.00 | 4.18 |
| train | 50 | 35.63 | 13.70 | 22.04 | 4.65 | |
| test | 30 | 26.47 | 16.39 | 21.86 | 2.36 | |
| S4 | all | 80 | 32.25 | 7.66 | 15.36 | 5.45 |
| train | 50 | 32.25 | 7.66 | 15.73 | 5.93 | |
| test | 30 | 20.69 | 8.20 | 14.24 | 3.55 | |
| All stages | all | 314 | 41.20 | 7.66 | 24.05 | 7.95 |
| train | 200 | 41.20 | 7.66 | 24.07 | 7.95 | |
| test | 114 | 37.46 | 8.20 | 24.00 | 8.00 |
| Bands Region | Correlation Order | Negative Correlation |
|---|---|---|
| 400–510 | S2 > S4 > S3 > S1 | None |
| 511–600 | |S2| > |S4| > |S3| > |S1| | All |
| 601–620 | |S4| > |S2| > |S3| > |S1| | All |
| 701–750 | |S2| > |S4| > |S3| > |S1| | All |
| 751–900 | S2 > S4 > S3 > |S1| | S1 |
| Wavelet Feature | Scale | Wavelengths | |R| |
|---|---|---|---|
| CA-WF1 | 21 | 572–573(2) | >0.71 |
| CA-WF2 | 21 | 734–737(4) | |
| CA-WF3 | 22 | 735–741(7) | |
| CA-WF4 | 22 | 964–966(3) | |
| CA-WF5 | 23 | 736–745(10) | |
| CA-WF6 | 24 | 745–749(5) | |
| CA-WF7 | 24 | 839 | |
| CA-WF8 | 25 | 763–769(7) | |
| CA-WF9 | 26 | 741–761(21) |
| Model | Variables | g | c | Acv (%) | Atrain (%) | Atrain − Acv (%) | Atest (%) |
|---|---|---|---|---|---|---|---|
| CA-SVM | 40 | 0.25 | 1024.00 | 78.00 | 88.50 | 10.50 | 76.32 |
| SPA-SVM | 36 | 0.03 | 337.79 | 90.50 | 99.00 | 8.80 | 92.11 |
| RF-SVM | 29 | 0.045 | 1024.00 | 96.25 | 98.75 | 2.50 | 94.59 |
| CA-CWT-SVM | 75 | 0.14 | 194.01 | 90.00 | 97.50 | 7.50 | 92.11 |
| SPA-CWT-SVM | 40 | 0.63 | 71.46 | 99.00 | 100.00 | 1.00 | 97.37 |
| RF-CWT-SVM | 36 | 0.44 | 36.75 | 94.00 | 99.50 | 5.50 | 94.74 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Zhao, R.; Qiao, L.; Zhang, Y.; Li, M.; Sun, H.; Xing, Z.; Wang, X. Growth Stages Classification of Potato Crop Based on Analysis of Spectral Response and Variables Optimization. Sensors 2020, 20, 3995. https://doi.org/10.3390/s20143995
Liu N, Zhao R, Qiao L, Zhang Y, Li M, Sun H, Xing Z, Wang X. Growth Stages Classification of Potato Crop Based on Analysis of Spectral Response and Variables Optimization. Sensors. 2020; 20(14):3995. https://doi.org/10.3390/s20143995
Chicago/Turabian StyleLiu, Ning, Ruomei Zhao, Lang Qiao, Yao Zhang, Minzan Li, Hong Sun, Zizheng Xing, and Xinbing Wang. 2020. "Growth Stages Classification of Potato Crop Based on Analysis of Spectral Response and Variables Optimization" Sensors 20, no. 14: 3995. https://doi.org/10.3390/s20143995