Fluorescence Anisotropy Sensor Comprising a Dual Hollow-Core Antiresonant Fiber Polarization Beam Splitter
Abstract
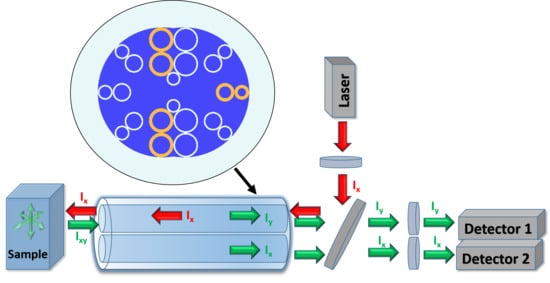
:1. Introduction
2. Polarization Beam Splitter
3. DHC-ARF-Based Fluorescence Anisotropy Sensor
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Debord, B.; Amsanpally, A.; Chafer, M.; Baz, A.; Maurel, M.; Blondy, J.M.; Hugonnot, E.; Scol, F.; Vincetti, L.; Gérôme, F.; et al. Ultralow transmission loss in inhibited-coupling guiding hollow fibers. Optica 2017, 4, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Bufetov, I.; Kosolapov, A.; Pryamikov, A.; Gladyshev, A.; Kolyadin, A.; Krylov, A.; Yatsenko, Y.; Biriukov, A. Revolver hollow core optical fibers. Fibers 2018, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Markos, C.; Travers, J.C.; Abdolvand, A.; Eggleton, B.J.; Bang, O. Hybrid photonic-crystal fiber. Rev. Mod. Phys. 2017, 89, 045003. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Ma, J.; Tang, D.; Yoo, S. Hollow-core air-gap anti-resonant fiber couplers. Opt. Express 2017, 25, 29296–29306. [Google Scholar] [CrossRef]
- Liu, X.; Fan, Z.; Shi, Z.; Ma, Y.; Yu, J.; Zhang, J. Dual-core antiresonant hollow core fibers. Opt. Express 2016, 24, 17453–17458. [Google Scholar] [CrossRef]
- Huang, X.; Yong, K.-T.; Yoo, S. A method to process hollow-core anti-resonant fibers into fiber filters. Fibers 2018, 6, 89. [Google Scholar] [CrossRef] [Green Version]
- Nissen, M.; Doherty, B.; Hamperl, J.; Kobelke, J.; Weber, K.; Henkel, T.; Schmidt, A.M. Uv absorption spectroscopy in water-filled antiresonant hollow core fibers for pharmaceutical detection. Sensors 2018, 18, 478. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-L.; Ding, W.; Wang, Y.-Y.; Gao, S.-F.; Cao, L.; Feng, X.; Wang, P. Characterization of a liquid-filled nodeless anti-resonant fiber for biochemical sensing. Opt. Lett. 2017, 42, 863–866. [Google Scholar] [CrossRef]
- Popenda, M.A.; Stawska, H.I.; Mazur, L.M.; Jakubowski, K.; Kosolapov, A.; Kolyadin, A.; Bereś-Pawlik, E. Application of negative curvature hollow-core fiber in an optical fiber sensor setup for multiphoton spectroscopy. Sensors 2017, 17, 2278. [Google Scholar] [CrossRef]
- Popenda, M.A.; Stawska, H.I.; Syperek, M.; Kosolapov, A.F.; Kolyadin, A.N.; Bereś-Pawlik, E. Multiphoton fluorescence excitation and detection with a single negative curvature hollow core fibre. Laser Phys. Lett. 2018, 16, 015103. [Google Scholar] [CrossRef]
- Lombardini, A.; Mytskaniuk, V.; Sivankutty, S.; Andresen, E.R.; Chen, X.; Wenger, J.; Fabert, M.; Joly, N.; Louradour, F.; Kudlinski, A.; et al. High-resolution multimodal flexible coherent raman endoscope. Light Sci. Appl. 2018, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Kudlinski, A.; Cassez, A.; Vanvincq, O.; Septier, D.; Pastre, A.; Habert, R.; Baudelle, K.; Douay, M.; Mytskaniuk, V.; Tsvirkun, V.; et al. Double clad tubular anti-resonant hollow core fiber for nonlinear microendoscopy. Opt. Express 2020, 28, 15062–15070. [Google Scholar] [CrossRef] [PubMed]
- Zolmajd-Haghighi, Z.; Hanley, Q.S. When one plus one does not equal two: Fluorescence anisotropy in aggregates and multiply labeled proteins. Biophys. J. 2014, 106, 1457–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, V.; Panicker, M.M.; Udgaonkar, J.B. Fluorescence anisotropy uncovers changes in protein packing with inclusion growth in a cellular model of polyglutamine aggregation. Biochemistry 2014, 53, 3621–3636. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.; Suhling, K.; Lévêque-Fort, S.; Webb, S.E.D.; Davis, D.M.; Phillips, D.; Sabharwal, Y.; French, P.M.W. Wide-field time-resolved fluorescence anisotropy imaging (tr-faim): Imaging the rotational mobility of a fluorophore. Rev. Sci. Instrum. 2003, 74, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Bader, A.N.; Hofman, E.G.; Voortman, J.; van Bergen en Henegouwen, P.M.P.; Gerritsen, H.C. Homo-fret imaging enables quantification of protein cluster sizes with subcellular resolution. Biophys. J. 2009, 97, 2613–2622. [Google Scholar] [CrossRef] [Green Version]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.Springer: New York, NY, USA, 2006. [Google Scholar]
- Jameson, D.M.; Ross, J.A. Fluorescence polarization/anisotropy in diagnostics and imaging. Chem. Rev. 2010, 110, 2685–2708. [Google Scholar] [CrossRef] [Green Version]
- Jameson, D.M.; Seifried, S.E. Quantification of protein–protein interactions using fluorescence polarization. Methods 1999, 19, 222–233. [Google Scholar] [CrossRef]
- Vinegoni, C.; Feruglio, P.F.; Gryczynski, I.; Mazitschek, R.; Weissleder, R. Fluorescence anisotropy imaging in drug discovery. Adv. Drug Deliv. Rev. 2019, 151–152, 262–288. [Google Scholar] [CrossRef]
- Yan, Y.; Marriott, G. Analysis of protein interactions using fluorescence technologies. Curr. Opin. Chem. Biol. 2003, 7, 635–640. [Google Scholar] [CrossRef]
- Mann, T.L.; Krull, U.J. Fluorescence polarization spectroscopy in protein analysis. Analyst 2003, 128, 313–317. [Google Scholar] [CrossRef]
- Casamayou-Boucau, Y.; Ryder, A.G. Extended wavelength anisotropy resolved multidimensional emission spectroscopy (armes) measurements: Better filters, validation standards, and rayleigh scatter removal methods. Methods Appl. Fluoresc. 2017, 5, 037001. [Google Scholar] [CrossRef]
- Au-Gijsbers, A.; Au-Nishigaki, T.; Au-Sánchez-Puig, N. Fluorescence anisotropy as a tool to study protein-protein interactions. JoVE 2016, 116, e54640. [Google Scholar]
- Pu, Y.; Wang, W.B.; Das, B.B.; Achilefu, S.; Alfano, R.R. Time-resolved fluorescence polarization dynamics and optical imaging of cytate: A prostate cancer receptor-targeted contrast agent. Appl. Opt. 2008, 47, 2281–2289. [Google Scholar] [CrossRef] [Green Version]
- Deng, T.; Li, J.; Zhang, L.-L.; Jiang, J.-H.; Chen, J.-N.; Shen, G.-L.; Yu, R.-Q. A sensitive fluorescence anisotropy method for the direct detection of cancer cells in whole blood based on aptamer-conjugated near-infrared fluorescent nanoparticles. Biosens. Bioelectron. 2010, 25, 1587–1591. [Google Scholar] [CrossRef]
- Cao, Z.; Huang, C.-C.; Tan, W. Nuclease resistance of telomere-like oligonucleotides monitored in live cells by fluorescence anisotropy imaging. Anal. Chem. 2006, 78, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Valeur, B. Molecular fluorescence. Digit. Encycl. Appl. Phys. 2009, 477–531. [Google Scholar]
- Callis, P.R. The theory of two-photon-induced fluorescence anisotropy. In Topics in Fluorescence Spectroscopy: Volume 5: Nonlinear and Two-Photon-Induced Fluorescence; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2002; pp. 1–42. [Google Scholar]
- Vinegoni, C.; Dubach, J.M.; Feruglio, P.F.; Weissleder, R. Two-photon fluorescence anisotropy microscopy for imaging and direct measurement of intracellular drug target engagement. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blacker, T.S.; Nicolaou, N.; Duchen, M.R.; Bain, A.J. Polarized two-photon absorption and heterogeneous fluorescence dynamics in nad(p)h. J. Phys. Chem. B 2019, 123, 4705–4717. [Google Scholar] [CrossRef] [PubMed]
- Kierdaszuk, B. Fluorescence anisotropy of tyrosinate anion using one-, two- and three-photon excitation. J. Fluoresc. 2013, 23, 339–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leeder, J.M.; Andrews, D.L. A molecular theory for two-photon and three-photon fluorescence polarization. J. Chem. Phys. 2011, 134, 094503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakowicz, J.R.; Gryczynski, I. Three-photon excitation of fluorescence. In Applied Fluorescence in Chemistry, Biology and Medicine; Rettig, W., Strehmel, B., Schrader, S., Seifert, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 137–157. [Google Scholar]
- Garcia, M.; Gruev, V. Optical characterization of rigid endoscopes and polarization calibration methods. Opt. Express 2017, 25, 15713–15728. [Google Scholar] [CrossRef]
- Charanya, T.; York, T.; Bloch, S.; Sudlow, G.; Liang, K.; Garcia, M.; Akers, W.J.; Rubin, D.; Gruev, V.; Achilefu, S. Trimodal color-fluorescence-polarization endoscopy aided by a tumor selective molecular probe accurately detects flat lesions in colitis-associated cancer. J. Biomed. Opt. 2014, 19, 126002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Gu, M. Polarization anisotropy in fiber-optic second harmonic generation microscopy. Opt. Express 2008, 16, 5000–5006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Jia, H.; Lian, Z.; Benson, T.; Lou, S. Ultra-broadband dual hollow-core anti-resonant fiber polarization splitter. Opt. Fiber Technol. 2019, 53, 102005. [Google Scholar] [CrossRef]
- Stawska, H.I.; Popenda, M.A. A dual hollow core antiresonant optical fiber coupler based on a highly birefringent structure-numerical design and analysis. Fibers 2019, 7, 109. [Google Scholar] [CrossRef] [Green Version]
- Rajeswari, D.; Sivanantha Raja, A.; Selvendran, S. Design and analysis of polarization splitter based on dual-core photonic crystal fiber. Optik 2017, 144, 15–21. [Google Scholar] [CrossRef]
- Vo-Dinh, T. Biomedical Photonics Handbook. Fundamentals, Devices, and Techniques; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Shi, L.; Lu, L.; Harvey, G.; Harvey, T.; Rodríguez-Contreras, A.; Alfano, R.R. Label-free fluorescence spectroscopy for detecting key biomolecules in brain tissue from a mouse model of alzheimer’s disease. Sci. Rep. 2017, 7, 2599. [Google Scholar] [CrossRef] [Green Version]
- Survey of Capillary Profiles—Hilgenberg Gmbh. Available online: https://www.hilgenberg-gmbh.de/en/products/glass-capillaries/capillary-profiles/ (accessed on 26 May 2020).
- Chaudhuri, S.; Putten, L.D.V.; Poletti, F.; Sazio, P.J.A. Low loss transmission in negative curvature optical fibers with elliptical capillary tubes. J. Lightwave Technol. 2016, 34, 4228–4231. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.-F.; Wang, Y.-Y.; Ding, W.; Jiang, D.-L.; Gu, S.; Zhang, X.; Wang, P. Hollow-core conjoined-tube negative-curvature fibre with ultralow loss. Nat. Commun. 2018, 9, 2828. [Google Scholar] [CrossRef] [Green Version]
- Mollah, M.A.; Habib, M.S.; Habib, M.S. Novel hollow-core asymmetric conjoined-tube anti-resonant fiber for low-loss thz wave guidance. OSA Contin. 2020, 3, 1169–1176. [Google Scholar] [CrossRef]
- Habib, M.S.; Antonio-Lopez, J.E.; Markos, C.; Schülzgen, A.; Amezcua-Correa, R. Single-mode, low loss hollow-core anti-resonant fiber designs. Opt. Express 2019, 27, 3824–3836. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, M.; Liu, Y. Nested low-loss hollow core fiber. IEEE J. Sel. Top. Quantum Electron. 2020, 26, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Hasan, M.I.; Hassan, M.R.A.; Chang, W. Effect of the second ring of antiresonant tubes in negative-curvature fibers. Opt. Express 2020, 28, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Lumerical Mode Solutions. Available online: https://www.lumerical.com/tcad-products/mode/ (accessed on 27 June 2019).





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stawska, H.I.; Popenda, M.A. Fluorescence Anisotropy Sensor Comprising a Dual Hollow-Core Antiresonant Fiber Polarization Beam Splitter. Sensors 2020, 20, 3321. https://doi.org/10.3390/s20113321
Stawska HI, Popenda MA. Fluorescence Anisotropy Sensor Comprising a Dual Hollow-Core Antiresonant Fiber Polarization Beam Splitter. Sensors. 2020; 20(11):3321. https://doi.org/10.3390/s20113321
Chicago/Turabian StyleStawska, Hanna Izabela, and Maciej Andrzej Popenda. 2020. "Fluorescence Anisotropy Sensor Comprising a Dual Hollow-Core Antiresonant Fiber Polarization Beam Splitter" Sensors 20, no. 11: 3321. https://doi.org/10.3390/s20113321