The Use of the LanthaScreen TR-FRET CAR Coactivator Assay in the Characterization of Constitutive Androstane Receptor (CAR) Inverse Agonists
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
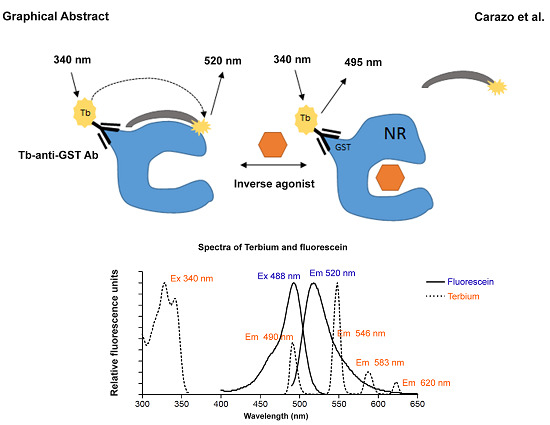
2.2. LanthaScreen TR-FRET Coactivator Assay

2.3. CAR LBD Assembly Assay
2.4. Data Analysis
3. Results and Discussion
3.1. Results
3.1.1. Optimization of TR-FRET Mixture Composition and Incubation Times

3.1.2. PK11195 Shows Competitive Inhibition for CAR

3.1.3. Androstenol does not Show Significant Competitive Inhibition for CAR LBD

3.1.4. Clotrimazole is a Potent Antagonist of Human CAR

3.1.5. Clotrimazole is a Potent Antagonist of Human CAR
| IC50 (μM)(95% Conf. Interv.)/R Square | |||
|---|---|---|---|
| TR- FRET CAR Assay | CAR LBD Assembly Assay | ||
| Compound | No CITCO | CITCO 1 μM | CITCO 1 μM |
| PK11195 | 0.51 (0.36–0.72) 0.998 | 93.63 (9.24–948.3) 0.858 | 56.61 (36.68–87.37) 0.905 |
| Androstenol | 0.345 (0.01–670.8) 0.968 | 312.6 (152.8–676.8) 0.318 | N.A. |
| Clotrimazole | 0.005 (0.0008–0.039) 0.968 | 6.15 (3.42–12.41) 0.932 | 11.37 (2.59–49.71) 0.865 |
3.2. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carazo Fernandez, A.; Smutny, T.; Hyrsova, L.; Berka, K.; Pavek, P. Chrysin, baicalein and galangin are indirect activators of the human constitutive androstane receptor (CAR). Toxicol. Lett. 2015, 233, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Zeng, S.; Xie, W. Nuclear receptors PXR and CAR: Implications for drug metabolism regulation, pharmacogenomics and beyond. Expert Opin. Drug Metab. Toxicol. 2013, 9, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Xie, W. Role of the constitutive androstane receptor in obesity and type 2 diabetes: A case study of the endobiotic function of a xenobiotic receptor. Drug Metab. Rev. 2013, 45, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Gao, J.; Xie, W. PXR and CAR in energy metabolism. Trends Endocr. Metab. 2009, 20, 273–279. [Google Scholar] [CrossRef]
- Ingraham, H.A.; Redinbo, M.R. Orphan nuclear receptors adopted by crystallography. Curr. Opin. Struct. Biol. 2005, 15, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Osabe, M.; Negishi, M. Active ERK1/2 protein interacts with the phosphorylated nuclear constitutive active/androstane receptor (CAR; NR1I3), repressing dephosphorylation and sequestering CAR in the cytoplasm. J. Biol. Chem. 2011, 286, 35763–35769. [Google Scholar] [CrossRef] [PubMed]
- Mutoh, S.; Sobhany, M.; Moore, R.; Perera, L.; Pedersen, L.; Sueyoshi, T.; Negishi, M. Phenobarbital indirectly activates the constitutive active androstane receptor (CAR) by inhibition of epidermal growth factor receptor signaling. Sci. Signal. 2013, 6, ra31. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H. Signaling control of the constitutive androstane receptor (CAR). Protein Cell 2014, 5, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Maglich, J.M.; Parks, D.J.; Moore, L.B.; Collins, J.L.; Goodwin, B.; Billin, A.N.; Stoltz, C.A.; Kliewer, S.A.; Lambert, M.H.; Willson, T.M.; Moore, J.T. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J. Biol. Chem. 2003, 278, 17277–17283. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.B.; Parks, D.J.; Jones, S.A.; Bledsoe, R.K.; Consler, T.G.; Stimmel, J.B.; Goodwin, B.; Liddle, C.; Blanchard, S.G.; Willson, T.M.; Collins, J.L.; Kliewer, S.A. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J. Biol. Chem. 2000, 275, 15122–15127. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, T.; Stanton, J.D.; Sueyoshi, T.; Negishi, M.; Wang, H. The peripheral benzodiazepine receptor ligand 1-(2-chlorophenyl-methylpropyl)-3-isoquinoline-carboxamide is a novel antagonist of human constitutive androstane receptor. Mol. Pharmacol. 2008, 74, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Forman, B.M.; Tzameli, I.; Choi, H.S.; Chen, J.; Simha, D.; Seol, W.; Evans, R.M.; Moore, D.D. Androstane metabolites bind to and deactivate the nuclear receptor CAR-beta. Nature 1998, 395, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Manufacturer’s_Manual, Invitrogen, Assay Pharmacology. In LanthaScreen™ TR-FRET Constitutive Androstane Receptor Coactivator Assay Manual; Invitrogen Corporation: Carlsbad, CA, USA, 2007.
- Kupcho, K.R.; Stafslien, D.K.; DeRosier, T.; Hallis, T.M.; Ozers, M.S.; Vogel, K.W. Simultaneous monitoring of discrete binding events using dual-acceptor terbium-based LRET. J. Am. Chem. Soc. 2007, 129, 13372–13373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T.; Williams, M. Defining and characterizing drug/compound function. Biochem. Pharmacol. 2014, 87, 40–63. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.J.; Blanchard, S.G.; Bledsoe, R.K.; Chandra, G.; Consler, T.G.; Kliewer, S.A.; Stimmel, J.B.; Willson, T.M.; Zavacki, A.M.; Moore, D.D.; et al. Bile acids: Natural ligands for an orphan nuclear receptor. Science 1999, 284, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.L.; Parks, D.J. Constitutive Androstane Receptor. Patent WO 2001071361 A2, 2001. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carazo, A.; Pávek, P. The Use of the LanthaScreen TR-FRET CAR Coactivator Assay in the Characterization of Constitutive Androstane Receptor (CAR) Inverse Agonists. Sensors 2015, 15, 9265-9276. https://doi.org/10.3390/s150409265
Carazo A, Pávek P. The Use of the LanthaScreen TR-FRET CAR Coactivator Assay in the Characterization of Constitutive Androstane Receptor (CAR) Inverse Agonists. Sensors. 2015; 15(4):9265-9276. https://doi.org/10.3390/s150409265
Chicago/Turabian StyleCarazo, Alejandro, and Petr Pávek. 2015. "The Use of the LanthaScreen TR-FRET CAR Coactivator Assay in the Characterization of Constitutive Androstane Receptor (CAR) Inverse Agonists" Sensors 15, no. 4: 9265-9276. https://doi.org/10.3390/s150409265