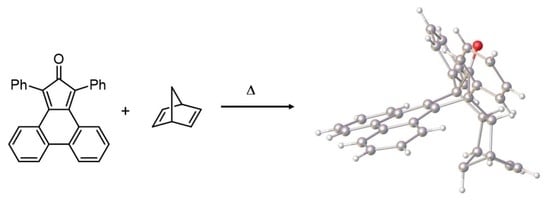
9,14-Diphenyl-9,9a,10,13,13a,14-hexahydro-9,14:10,13-dimethanobenzo[f]tetraphen-15-one
Abstract
:1. Introduction
2. Results
3. Experimental Section
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mackenzie, K. Bicyclo [2.2.1]heptadiene in the Diels-Alder Reaction. J. Chem. Soc. 1960, 473–483. [Google Scholar] [CrossRef]
- Sasaki, T.; Kanematsu, K.; Iizuka, K. Molecular design by cycloaddition reactions XXV. High peri- and regiospecificity of phencyclone. J. Org. Chem. 1976, 41, 1105–1112. [Google Scholar] [CrossRef]
- Harrison, E.A. Synthesis of a bicyclo[2.2.1]heptene Diels-Alder adduct: An organic chemistry experiment utilizing NMR spectroscopy to assign endo stereochemistry. J. Chem. Educ. 1991, 68, 426–427. [Google Scholar] [CrossRef]
- Smellie, I.A.; Carpenter-Warren, C.L.; Chalmers, B.A.; Cordes, D.B.; De, A.; Gouy, R.P.F.; Keddie, N.S.; Lebl, T.; Patterson, I.L.J.; Slawin, A.M.Z. Simple and inexpensive method for the detection of carbon monoxide released from thermal cheletropic decarbonylation reactions. J. Chem. Educ. 2021, 98, 3608–3613. [Google Scholar] [CrossRef]
- Callahan, R.; Ramirez, O.; Rosmarion, K.; Rothchild, R.; Bynum, K.C.; Multinuclear, N.M.R. Studies and ab initio structure calculations of hindered phencyclone Diels-Alder adducts from symmetrical cyclic dienophiles: Cyclohexene, vinylene carbonate, vinylene trithiocarbonate and two N-aryl maleimides. J. Heterocycl. Chem. 2005, 42, 889–898. [Google Scholar] [CrossRef]
- Blake, C.; Chalmers, B.A.; Clough, L.A.; Clunie, D.; Cordes, D.B.; Lebl, T.; McDonald, T.R.; Smith, S.R.; Stuart-Morrison, E.; Smellie, I.A. (9R,9aS,12aR,13S)-9,13-Diphenyl-9,9a,12a,13-tetrahydro-9,13-methanotriphenyleno[2,3-c]furan-10,12,14-trione. Molbank 2022, M1435. [Google Scholar] [CrossRef]
- Yit Wooi, G.; White, J.M. Structural Manifestations of the Cheletropic Reaction. Org. Biomol. Chem. 2005, 3, 972–974. [Google Scholar] [CrossRef] [PubMed]
- CrystalClear-SM Expert, v2.1; Rigaku Americas: The Woodlands, TX, USA; Rigaku Corporation: Tokyo, Japan, 2015.
- CrysAlisPro, v1.171.41.93a; Rigaku Oxford Diffraction; Rigaku Corporation: Oxford, UK, 2020.
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalmers, B.A.; Cordes, D.B.; Doig, T.; Du, Y.; Lebl, T.; Liu, M.; Mealyou, F.; Rainer, J.; Smith, S.R.; Walker, R.; et al. 9,14-Diphenyl-9,9a,10,13,13a,14-hexahydro-9,14:10,13-dimethanobenzo[f]tetraphen-15-one. Molbank 2022, 2022, M1524. https://doi.org/10.3390/M1524
Chalmers BA, Cordes DB, Doig T, Du Y, Lebl T, Liu M, Mealyou F, Rainer J, Smith SR, Walker R, et al. 9,14-Diphenyl-9,9a,10,13,13a,14-hexahydro-9,14:10,13-dimethanobenzo[f]tetraphen-15-one. Molbank. 2022; 2022(4):M1524. https://doi.org/10.3390/M1524
Chicago/Turabian StyleChalmers, Brian A., David B. Cordes, Thomas Doig, Yuanyuan Du, Tomas Lebl, Meiyue Liu, Fraser Mealyou, Jasmine Rainer, Siobhan R. Smith, Ryan Walker, and et al. 2022. "9,14-Diphenyl-9,9a,10,13,13a,14-hexahydro-9,14:10,13-dimethanobenzo[f]tetraphen-15-one" Molbank 2022, no. 4: M1524. https://doi.org/10.3390/M1524