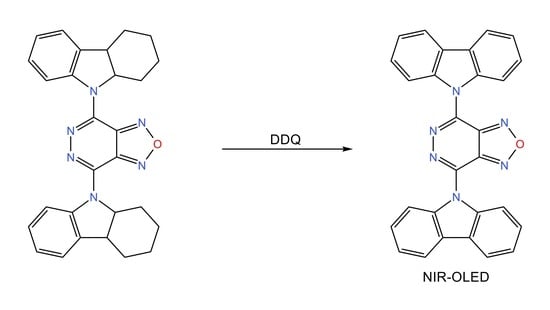
4,7-Di(9H-carbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yahya, M.; Bouziani, A.; Ocak, C.; Seferoğlu, Z.; Sillanpää, M. Organic/metal-organic photosensitizers for dye-sensitized solar cells (DSSC): Recent developments, new trends, and future perceptions. Dyes Pigm. 2021, 192, 109227. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Rakitin, O.A. Influence of structural factors on the photovoltaic properties of dye-sensitized solar cells. Russ. Chem. Rev. 2016, 85, 1146–1183. [Google Scholar] [CrossRef]
- Zhao, R.; Min, Y.; Dou, C.; Lin, B.; Ma, W.; Liu, J.; Wang, L. A Conjugated Polymer Containing a B ← N Unit for Unipolar n-Type Organic Field-Effect Transistors. ACS Appl. Polym. Mater. 2020, 2, 19–25. [Google Scholar] [CrossRef]
- Tang, Z.; Wei, X.; Zhang, W.; Zhou, Y.; Wei, C.; Huang, J.; Chen, Z.; Wang, L.; Yu, G. An A−D−A′−D′ strategy enables perylenediimide-based polymer dyes exhibiting enhanced electron transport characteristics. Polymer (Guildf) 2019, 180, 121712. [Google Scholar] [CrossRef]
- Stuart, A.C.; Tumbleston, J.R.; Zhou, H.; Li, W.; Liu, S.; Ade, H.; You, W. Fluorine Substituents Reduce Charge Recombination and Drive Structure and Morphology Development in Polymer Solar Cells. J. Am. Chem. Soc. 2013, 135, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, V.M.; Chmovzh, T.N.; Golovanov, I.S.; Knyazeva, E.A.; Mikhalchenko, L.V.; Saifutyarov, R.S.; Avetisov, I.C.; Woollins, J.D.; Taydakov, I.V.; Rakitin, O.A. Candle light-style OLEDs with benzochalcogenadiazoles cores. Dyes Pigm. 2021, 185, 108917. [Google Scholar] [CrossRef]
- Rakitin, O.A. Recent developments in the synthesis of 1,2,5-thiadiazoles and 2,1,3-benzothiadiazoles. Synthesis 2019, 51, 4338–4347. [Google Scholar] [CrossRef]
- Rakitin, O.A. 1,2,5-Thiadiazoles. In Comprehensive Heterocyclic Chemistry IV; Elsevier: Amsterdam, The Netherlands, 2022; pp. 371–406. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Knyazeva, E.A.; Tanaka, E.; Popov, V.V.; Mikhalchenko, L.V.; Robertson, N.; Rakitin, O.A. [1,2,5]Thiadiazolo [3,4-d]Pyridazine as an Internal Acceptor in the D-A-π-A Organic Sensitizers for Dye-Sensitized Solar Cells. Molecules 2019, 24, 1588. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, V.M.; Chmovzh, T.N.; Chkhetiani, G.R.; Taydakov, I.V.; Rakitin, O.A. New D–A–D luminophores of the [1,2,5]thiadiazolo[3,4-d]pyridazine series. Mendeleev Commun. 2022, 32, 371–373. [Google Scholar] [CrossRef]
- Leventis, A.; Chmovzh, T.N.; Knyazeva, E.A.; Han, Y.; Heeney, M.; Rakitin, O.A.; Bronstein, H. A novel low-bandgap pyridazine thiadiazole-based conjugated polymer with deep molecular orbital levels. Polym. Chem. 2020, 11, 581–585. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Ding, W.; Zhao, X.; Geng, Z. Density functional theory design and characterization of D–A–A type electron donors with narrow band gap for small-molecule organic solar cells. Comput. Theor. Chem. 2014, 1029, 68–78. [Google Scholar] [CrossRef]
- Chochos, C.L.; Choulis, S.A. How the structural deviations on the backbone of conjugated polymers influence their optoelectronic properties and photovoltaic performance. Prog. Polym. Sci. 2011, 36, 1326–1414. [Google Scholar] [CrossRef]
- Wang, K.; Fu, X.; Tang, Q.; Li, H.; Shu, Y.; Li, J.; Pang, W. Theoretical investigations on novel energetic salts composed of 4-nitro-7-(4-nitro-1,2,3-triazol-1-olate)-furazano [3,4-d]pyridazine-based anions and ammonium-based cations. Comput. Mater. Sci. 2018, 146, 230–239. [Google Scholar] [CrossRef]
- Wang, K.; Shu, Y.; Liu, N.; Lai, W.; Yu, T.; Ding, X.; Wu, Z. Theoretical studies on structure and performance of [1,2,5]-oxadiazolo-[3,4-d]-pyridazine-based derivatives. J. Phys. Org. Chem. 2017, 30, e3591. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Gaisin, K.S.; Rakitin, O.A. 4,7-Bis(1,2,3,4,4a,9a-Hexahydro-9H-carbazol-9-yl)-[1,2,5]oxadiazolo [3,4-d]pyridazine. Molbank 2021, 2021, M1295. [Google Scholar] [CrossRef]
- Chmovzh, T.; Knyazeva, E.; Lyssenko, K.; Popov, V.; Rakitin, O. Safe Synthesis of 4,7-Dibromo [1,2,5]thiadiazolo [3,4-d]pyridazine and Its SNAr Reactions. Molecules 2018, 23, 2576. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xiao, Y.; Chi, S.; Qian, X. Isomeric Boron−Fluorine Complexes with Donor−Acceptor Architecture: Strong Solid/Liquid Fluorescence and Large Stokes Shift. Org. Lett. 2008, 10, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Santa, T.; Imai, K. Fluorescence characteristics of six 4,7-disubstituted benzofurazan compounds: An experimental and semi-empirical MO study. J. Chem. Soc. Perkin Trans. 2 1999, 2525–2532. [Google Scholar] [CrossRef]

| Solvent | λabs(LE) nm | λabs(ICT) nm | εmax mol × 1−1 × cm−1 | λem nm (cm−1) | Stokes Shift ∆ν cm−1 |
|---|---|---|---|---|---|
| CHCl3 | 321 | 530 | 9710 | 706 (14,164) | 4700 |
| THF | 321 | 501 | 9190 | 659 (15,174) | 4790 |
| DMSO | 323 | 499 | 8850 | - | - |
| MeCN | 321 | 503 | 9580 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmovzh, T.N.; Kudryashev, T.A.; Gaisin, K.S.; Rakitin, O.A. 4,7-Di(9H-carbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine. Molbank 2022, 2022, M1428. https://doi.org/10.3390/M1428
Chmovzh TN, Kudryashev TA, Gaisin KS, Rakitin OA. 4,7-Di(9H-carbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine. Molbank. 2022; 2022(3):M1428. https://doi.org/10.3390/M1428
Chicago/Turabian StyleChmovzh, Timofey N., Timofey A. Kudryashev, Karim S. Gaisin, and Oleg A. Rakitin. 2022. "4,7-Di(9H-carbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine" Molbank 2022, no. 3: M1428. https://doi.org/10.3390/M1428