Design of Mixed Medicinal Plants, Rich in Polyphenols, Vitamins B, and Palmitoylethanolamide-Based Supplement to Help Reduce Nerve Pain: A Preclinical Study
Abstract
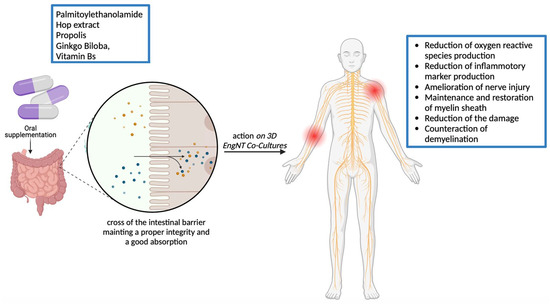
:1. Introduction
2. Results
2.1. Dose-Response Assessment Study of Single Components on CaCo-2 Cells
2.2. Functional Assessment at Intestinal Level
2.3. Effects of Single Components and Combination on 3D EngNT Cocultures
3. Discussion
4. Materials and Methods
4.1. Agents Preparation
4.2. Cell Culture
4.3. Experimental Procedure
4.4. In Vitro Simulation of Intestinal Barrier
4.5. 3D EngNT Co-Cultures Setup
4.6. Cell Viability
4.7. ROS Production
4.8. TJs Analysis
4.9. SOD Assay
4.10. Glutathione Peroxidase Assay
4.11. TNFα Assay
4.12. NRG1 Assay
4.13. Myelin Protein Zero Assay
4.14. NGFR Assay (p75 Expression Assay)
4.15. Estrogen Receptor Beta Assay
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCI | chronic nerve constriction injury |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMEM-Adv | Dulbecco’s Modified Eagle’s Medium Advance |
| ERb | epidermal receptor beta |
| EMA | European Medicines Agency |
| FBS | foetal bovine serum |
| FDA | Food and Drug Administration |
| GGF | glial growth factor 2 |
| HS | horse serum |
| MEM | Minimum Essential Medium |
| MPZ | myelin protein zero |
| NP | neuropathic pain |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| Papp | apparent permeability coefficient |
| PCPA | P-chlorophenylalanine |
| PEA | palmitoylethanolamide |
| ROS | reactive oxygen species |
| RPMI-Adv | Roswell Park Memorial Institute-1640 Advance |
| SOD | superoxide dismutase |
| TJ | tight junction |
| TEER | transepithelial electrical resistance |
| TNFα | tumor necrosis factor α |
| 6-OHDA | 6-hydroxydopamine |
| ZO-1 | Zonula occludens-1 |
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 789–858. [Google Scholar]
- Treede, R.D.; Jensen, T.S.; Campbell, J.N.; Cruccu, G.; Dostrovsky, J.O.; Griffin, J.W.; Hansson, P.; Hughes, R.; Nurmikko, T.; Serra, J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 2008, 70, 1630–1635. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Baskozos, G.; Hébert, H.L.; Pascal, M.M.; Themistocleous, A.C.; Macfarlane, G.J.; Wynick, D.; Bennett, D.L.; Smith, B.H. Epidemiology of neuropathic pain: An analysis of prevalence and associated factors in UK Biobank. Pain Rep. 2023, 8, e1066. [Google Scholar] [CrossRef] [PubMed]
- Bronge, W.; Lindholm, B.; Elmståhl, S.; Siennicki-Lantz, A. Epidemiology and functional impact of early peripheral neuropathy signs in older adults from a general population. Gerontology 2023, 70, 257–268. [Google Scholar] [CrossRef]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef]
- Vanneste, S.; To, W.T.; De Ridder, D. Tinnitus and neuropathic pain share a common neural substrate in the form of specific brain connectivity and microstate profiles. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 88, 388–400. [Google Scholar] [CrossRef]
- Baron, R.; Binder, A.; Wasner, G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010, 9, 807–819. [Google Scholar] [CrossRef]
- Bouhassira, D.; Attal, N.; Fermanian, J.; Alchaar, H.; Gautron, M.; Masquelier, E.; Rostaing, S.; Lanteri-Minet, M.; Collin, E.; Grisart, J.; et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain 2004, 108, 248–257. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.L.H.; Bouhassira, D.; Cruccu, G.; Freeman, R.; Hansson, P.; Nurmikko, T.; et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain 2016, 157, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Saporta, M.A.; Shy, M.E. Chapter 12—Peripheral neuropathies. In Neurobiology of Brain Disorders; Zigmond, M.J., Rowland, L.P., Coyle, J.T., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 167–188. [Google Scholar]
- Mallet, M.L.; Hadjivassiliou, M.; Sarrigiannis, P.G.; Zis, P. The Role of Oxidative Stress in Peripheral Neuropathy. J. Mol. Neurosci. 2020, 70, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.K.; Tandan, S.K.; Dudhgaonkar, S.P.; Jadhav, S.H.; Kataria, M.; Prakash, V.R.; Kumar, D. Role of oxidative stress in pathophysiology of peripheral neuropathy and modulation by N-acetyl-L-cysteine in rats. Eur. J. Pain 2006, 10, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Neri, S.; Signorelli, S.; Pulvirenti, D.; Mauceri, B.; Cilio, D.; Bordonaro, F.; Abate, G.; Interlandi, D.; Misseri, M.; Ignaccolo, L.; et al. Oxidative stress, nitric oxide, endothelial dysfunction and tinnitus. Free Radic. Res. 2006, 40, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Pawlak-Osińska, K.; Kaźmierczak, H.; Marzec, M.; Kupczyk, D.; Bilski, R.; Mikołajewska, E.; Mikołajewski, D.; Augustyńska, B. Assessment of the State of the Natural Antioxidant Barrier of a Body in Patients Complaining about the Presence of Tinnitus. Oxid. Med. Cell. Longev. 2018, 2018, 1439575. [Google Scholar] [CrossRef] [PubMed]
- El Soury, M.; Fornasari, B.E.; Carta, G.; Zen, F.; Haastert-Talini, K.; Ronchi, G. The Role of Dietary Nutrients in Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2021, 22, 7417. [Google Scholar] [CrossRef]
- Haase, G.M.; Prasad, K.N.; Cole, W.C.; Baggett-Strehlau, J.M.; Wyatt, S.E. Antioxidant micronutrient impact on hearing disorders: Concept, rationale, and evidence. Am. J. Otolaryngol. 2011, 32, 55–61. [Google Scholar] [CrossRef]
- Boyd, A.; Bleakley, C.; Hurley, D.A.; Gill, C.; Hannon-Fletcher, M.; Bell, P.; McDonough, S. Herbal medicinal products or preparations for neuropathic pain. Cochrane Database Syst. Rev. 2019, 4, CD010528. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Adams, J.D. Treatment of neuropathic pain with plant medicines. Chin. J. Integr. Med. 2012, 18, 565–570. [Google Scholar] [CrossRef]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Dagostino, C.; Gliozzi, M.; Malafoglia, V.; Sansone, L.; Palma, E.; Tafani, M.; Russo, M.A.; et al. Antioxidant modulation of sirtuin 3 during acute inflammatory pain: The ROS control. Pharmacol. Res. 2020, 157, 104851. [Google Scholar] [CrossRef]
- Lauro, F.; Giancotti, L.A.; Ilari, S.; Dagostino, C.; Gliozzi, M.; Morabito, C.; Malafoglia, V.; Raffaeli, W.; Muraca, M.; Goffredo, B.M.; et al. Inhibition of Spinal Oxidative Stress by Bergamot Polyphenolic Fraction Attenuates the Development of Morphine Induced Tolerance and Hyperalgesia in Mice. PLoS ONE 2016, 11, e0156039. [Google Scholar] [CrossRef]
- Rahman, I.; Chung, S. Dietary polyphenols, deacetylases and chromatin remodeling in inflammation. J. Nutr. Nutr. 2010, 3, 220–230. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, H.J.; Kim, T.K.; Moon, D.E.; Lee, H.J. The effects of Ginkgo biloba extract EGb 761 on mechanical and cold allodynia in a rat model of neuropathic pain. Anesth. Analg. 2009, 108, 1958–1963. [Google Scholar] [CrossRef]
- Al-Adwani, D.G.; Renno, W.M.; Orabi, K.Y. Neurotherapeutic effects of Ginkgo biloba extract and its terpene trilactone, ginkgolide B, on sciatic crush injury model: A new evidence. PLoS ONE 2019, 14, e0226626. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Dong, J.; Lv, W.; Zhao, S.; Jin, L.; Guo, J.; Wang, M.; Cai, C.; Sun, J.; et al. Effects of Ginkgo biloba on Early Decompression after Spinal Cord Injury. Evid. Based Complement. Alternat Med. 2020, 2020, 6958246. [Google Scholar] [CrossRef]
- Ekici-Günay, N. Chapter 23—Ginkgo biloba extract as an antioxidant in nerve regeneration. In Pathology; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Hallak, B.; Schneider, A.; Güntensperger, D.; Schapowal, A. Standardized Ginkgo biloba Extract in the Treatment of Vertigo and/or Tinnitus: A Review of the Literature. Adv. Aging Res. 2021, 10, 31. [Google Scholar] [CrossRef]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Gliozzi, M.; Malafoglia, V.; Palma, E.; Tafani, M.; Russo, M.A.; Tomino, C.; Fini, M.; et al. Natural Antioxidant Control of Neuropathic Pain-Exploring the Role of Mitochondrial SIRT3 Pathway. Antioxidants 2020, 9, 1103. [Google Scholar] [CrossRef]
- Nowak, A.; Kojder, K.; Zielonka-Brzezicka, J.; Wróbel, J.; Bosiacki, M.; Fabiańska, M.; Wróbel, M.; Sołek-Pastuszka, J.; Klimowicz, A. The Use of Ginkgo Biloba L. as a Neuroprotective Agent in the Alzheimer’s Disease. Front. Pharmacol. 2021, 12, 775034. [Google Scholar] [CrossRef]
- Liu, X.P.; Goldring, C.E.; Copple, I.M.; Wang, H.Y.; Wei, W.; Kitteringham, N.R.; Park, B.K. Extract of Ginkgo biloba induces phase 2 genes through Keap1-Nrf2-ARE signaling pathway. Life Sci. 2007, 80, 1586–1591. [Google Scholar] [CrossRef]
- Bolton, J.L.; Dunlap, T.L.; Hajirahimkhan, A.; Mbachu, O.; Chen, S.N.; Chadwick, L.; Nikolic, D.; van Breemen, R.B.; Pauli, G.F.; Dietz, B.M. The Multiple Biological Targets of Hops and Bioactive Compounds. Chem. Res. Toxicol. 2019, 32, 222–233. [Google Scholar] [CrossRef]
- Park, S.H.; Sim, Y.B.; Kang, Y.J.; Kim, S.S.; Kim, C.H.; Kim, S.J.; Seo, J.Y.; Lim, S.M.; Suh, H.W. Hop extract produces antinociception by acting on opioid system in mice. Korean J. Physiol. Pharmacol. 2012, 16, 187–192. [Google Scholar] [CrossRef]
- Julian, T.; Syeed, R.; Glascow, N.; Angelopoulou, E.; Zis, P. B12 as a Treatment for Peripheral Neuropathic Pain: A Systematic Review. Nutrients 2020, 12, 2221. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, Y.H. Relationship between Diet and Tinnitus: Korea National Health and Nutrition Examination Survey. Clin. Exp. Otorhinolaryngol. 2018, 11, 158–165. [Google Scholar] [CrossRef]
- Petrosino, S.; Iuvone, T.; Di Marzo, V. N-palmitoyl-ethanolamine: Biochemistry and new therapeutic opportunities. Biochimie 2010, 92, 724–727. [Google Scholar] [CrossRef]
- Zulhendri, F.; Perera, C.O.; Tandean, S. Can Propolis Be a Useful Adjuvant in Brain and Neurological Disorders and Injuries? A Systematic Scoping Review of the Latest Experimental Evidence. Biomedicines 2021, 9, 1227. [Google Scholar] [CrossRef]
- Nosratiyan, M.; Farjah, G.H.; Karimipour, M.; Pourheidar, B. The Effect of Propolis-Gum Arabic as a Novel Nerve Guidance Channel on Regeneration of Sciatic Nerve in Male Rats. Turk. Neurosurg. 2021, 31, 361–367. [Google Scholar] [CrossRef]
- Ilari, S.; Proietti, S.; Russo, P.; Malafoglia, V.; Gliozzi, M.; Maiuolo, J.; Oppedisano, F.; Palma, E.; Tomino, C.; Fini, M.; et al. A Systematic Review and Meta-Analysis on the Role of Nutraceuticals in the Management of Neuropathic Pain in In Vivo Studies. Antioxidants 2022, 11, 2361. [Google Scholar] [CrossRef]
- Gierthmühlen, J.; Baron, R. Neuropathic Pain. Semin. Neurol. 2016, 36, 462–468. [Google Scholar] [CrossRef]
- Cohen, S.P.; Mao, J. Neuropathic pain: Mechanisms and their clinical implications. BMJ 2014, 348, f7656. [Google Scholar] [CrossRef]
- Jeong, J.-Y.; Jung, I.-G.; Yum, S.-H.; Hwang, Y.-J. In Vitro Synergistic Inhibitory Effects of Plant Extract Combinations on Bacterial Growth of Methicillin-Resistant Staphylococcus aureus. Pharmaceuticals 2023, 16, 1491. [Google Scholar] [CrossRef]
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically Active Compounds from Hops and Prospects for Their Use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Strycharz-Dudziak, M.; Fołtyn, S.; Dworzański, J.; Kiełczykowska, M.; Malm, M.; Drop, B.; Polz-Dacewicz, M. Glutathione Peroxidase (GPx) and Superoxide Dismutase (SOD) in Oropharyngeal Cancer Associated with EBV and HPV Coinfection. Viruses 2020, 12, 1008. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Myers, R.R. Upregulation and interaction of TNFalpha and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000, 855, 83–89. [Google Scholar] [CrossRef]
- Ohtori, S.; Takahashi, K.; Moriya, H.; Myers, R.R. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: Studies in murine DRG and spinal cord. Spine 2004, 29, 1082–1088. [Google Scholar] [CrossRef]
- Sommer, C.; Schäfers, M.; Marziniak, M.; Toyka, K.V. Etanercept reduces hyperalgesia in experimental painful neuropathy. J. Peripher. Nerv. Syst. 2001, 6, 67–72. [Google Scholar] [CrossRef]
- Goldie, M.; Dolan, S. Bilobalide, a unique constituent of Ginkgo biloba, inhibits inflammatory pain in rats. Behav. Pharmacol. 2013, 24, 298–306. [Google Scholar] [CrossRef]
- Darmani, N.A.; Izzo, A.A.; Degenhardt, B.; Valenti, M.; Scaglione, G.; Capasso, R.; Sorrentini, I.; Di Marzo, V. Involvement of the cannabimimetic compound, N-palmitoyl-ethanolamine, in inflammatory and neuropathic conditions: Review of the available pre-clinical data, and first human studies. Neuropharmacology 2005, 48, 1154–1163. [Google Scholar] [CrossRef]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef]
- Newbern, J.; Birchmeier, C. Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin. Cell Dev. Biol. 2010, 21, 922–928. [Google Scholar] [CrossRef]
- Pan, P.; Dobrowsky, R.T. Differential expression of neuregulin-1 isoforms and downregulation of erbin are associated with Erb B2 receptor activation in diabetic peripheral neuropathy. Acta Neuropathol. Commun. 2013, 1, 39. [Google Scholar] [CrossRef]
- Ibáñez, C.F.; Simi, A. p75 neurotrophin receptor signaling in nervous system injury and degeneration: Paradox and opportunity. Trends Neurosci. 2012, 35, 431–440. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Y.; Wu, S.; Zhao, M.Y. Review: Myelin clearance is critical for regeneration after peripheral nerve injury. Front. Neurol. 2022, 13, 908148. [Google Scholar] [CrossRef]
- Morsanuto, V.; Galla, R.; Molinari, C.; Uberti, F. A New Palmitoylethanolamide Form Combined with Antioxidant Molecules to Improve Its Effectivess on Neuronal Aging. Brain Sci. 2020, 10, 457. [Google Scholar] [CrossRef]
- Bessa Pereira, C.; Gomes, P.S.; Costa-Rodrigues, J.; Almeida Palmas, R.; Vieira, L.; Ferraz, M.P.; Lopes, M.A.; Fernandes, M.H. Equisetum arvense hydromethanolic extracts in bone tissue regeneration: In vitro osteoblastic modulation and antibacterial activity. Cell Prolif. 2012, 45, 386–396. [Google Scholar] [CrossRef]
- Lea, T. Caco-2 cell line. In The Impact of Food Bioactives on Health; Springer: Cham, Switzerland, 2015; pp. 103–111. [Google Scholar]
- DiMarco, R.L.; Hunt, D.R.; Dewi, R.E.; Heilshorn, S.C. Improvement of paracellular transport in the Caco-2 drug screening model using protein-engineered substrates. Biomaterials 2017, 129, 152–162. [Google Scholar] [CrossRef]
- Galla, R.; Ruga, S.; Aprile, S.; Ferrari, S.; Brovero, A.; Grosa, G.; Molinari, C.; Uberti, F. New Hyaluronic Acid from Plant Origin to Improve Joint Protection-An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 8114. [Google Scholar] [CrossRef]
- Uberti, F.; Morsanuto, V.; Ruga, S.; Galla, R.; Farghali, M.; Notte, F.; Bozzo, C.; Magnani, C.; Nardone, A.; Molinari, C. Study of Magnesium Formulations on Intestinal Cells to Influence Myometrium Cell Relaxation. Nutrients 2020, 12, 573. [Google Scholar] [CrossRef]
- Uberti, F.; Trotta, F.; Pagliaro, P.; Bisericaru, D.M.; Cavalli, R.; Ferrari, S.; Penna, C.; Matencio, A. Developing New Cyclodextrin-Based Nanosponges Complexes to Improve Vitamin D Absorption in an In Vitro Study. Int. J. Mol. Sci. 2023, 24, 5322. [Google Scholar] [CrossRef]
- Rayner, M.L.D.; Laranjeira, S.; Evans, R.E.; Shipley, R.J.; Healy, J.; Phillips, J.B. Developing an In Vitro Model to Screen Drugs for Nerve Regeneration. Anat. Rec. 2018, 301, 1628–1637. [Google Scholar] [CrossRef]
- Muscella, A.; Vetrugno, C.; Cossa, L.G.; Marsigliante, S. TGF-β1 activates RSC96 Schwann cells migration and invasion through MMP-2 and MMP-9 activities. J. Neurochem. 2020, 153, 525–538. [Google Scholar] [CrossRef]
- Lahiani, A.; Brand-Yavin, A.; Yavin, E.; Lazarovici, P. Neuroprotective Effects of Bioactive Compounds and MAPK Pathway Modulation in "Ischemia"-Stressed PC12 Pheochromocytoma Cells. Brain Sci. 2018, 8, 32. [Google Scholar] [CrossRef]
- Chua, P.; Lim, W.K. Optimisation of a PC12 cell-based in vitro stroke model for screening neuroprotective agents. Sci. Rep. 2021, 11, 8096. [Google Scholar] [CrossRef] [PubMed]
- Galla, R.; Grisenti, P.; Farghali, M.; Saccuman, L.; Ferraboschi, P.; Uberti, F. Ovotransferrin Supplementation Improves the Iron Absorption: An In Vitro Gastro-Intestinal Model. Biomedicines 2021, 9, 1543. [Google Scholar] [CrossRef]
- Park, S.E.; Ahn, J.; Jeong, H.-E.; Youn, I.; Huh, D.; Chung, S. A three-dimensional in vitro model of the peripheral nervous system. NPG Asia Mater. 2021, 13, 2. [Google Scholar] [CrossRef]
- Uberti, F.; Morsanuto, V.; Ghirlanda, S.; Molinari, C. Iron Absorption from Three Commercially Available Supplements in Gastrointestinal Cell Lines. Nutrients 2017, 9, 1008. [Google Scholar] [CrossRef]
- Christides, T.; Wray, D.; McBride, R.; Fairweather, R.; Sharp, P. Iron bioavailability from commercially available iron supplements. Eur. J. Nutr. 2015, 54, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Fda.Gov. Available online: https://www.fda.gov/media/117974/download (accessed on 12 September 2023).
- Ema.Eu. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-m9-biopharmaceutics-classification-system-based-biowaivers-step-2b-first-version_en.pdf (accessed on 12 September 2023).
- Guha, S.; Alvarez, S.; Majumder, K. Transport of Dietary Anti-Inflammatory Peptide, γ-Glutamyl Valine (γ-EV), across the Intestinal Caco-2 Monolayer. Nutrients 2021, 13, 1448. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
- Schuh, C.M.A.P.; Day, A.G.E.; Redl, H.; Phillips, J. An Optimized Collagen-Fibrin Blend Engineered Neural Tissue Promotes Peripheral Nerve Repair. Tissue Eng. Part A 2018, 24, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Muangsanit, P.; Day, A.; Dimiou, S.; Ataç, A.F.; Kayal, C.; Park, H.; Nazhat, S.N.; Phillips, J.B. Rapidly formed stable and aligned dense collagen gels seeded with Schwann cells support peripheral nerve regeneration. J. Neural Eng. 2020, 17, 046036. [Google Scholar] [CrossRef] [PubMed]
- Uberti, F.; Ruga, S.; Farghali, M.; Galla, R.; Molinari, C. A Combination of α-Lipoic Acid (ALA) and Palmitoylethanolamide (PEA) Blocks Endotoxin-Induced Oxidative Stress and Cytokine Storm: A Possible Intervention for COVID-19. J. Diet. Suppl. 2023, 20, 133–155. [Google Scholar] [CrossRef] [PubMed]
- Ruga, S.; Galla, R.; Ferrari, S.; Invernizzi, M.; Uberti, F. Novel Approach to the Treatment of Neuropathic Pain Using a Combination with Palmitoylethanolamide and. Int. J. Mol. Sci. 2023, 24, 5503. [Google Scholar] [CrossRef]
- Rzemieniec, J.; Litwa, E.; Wnuk, A.; Lason, W.; Gołas, A.; Krzeptowski, W.; Kajta, M. Neuroprotective action of raloxifene against hypoxia-induced damage in mouse hippocampal cells depends on ERα but not ERβ or GPR30 signalling. J. Steroid Biochem. Mol. Biol. 2015, 146, 26–37. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulè, S.; Rosso, G.; Botta, M.; Brovero, A.; Ferrari, S.; Galla, R.; Molinari, C.; Uberti, F. Design of Mixed Medicinal Plants, Rich in Polyphenols, Vitamins B, and Palmitoylethanolamide-Based Supplement to Help Reduce Nerve Pain: A Preclinical Study. Int. J. Mol. Sci. 2024, 25, 4790. https://doi.org/10.3390/ijms25094790
Mulè S, Rosso G, Botta M, Brovero A, Ferrari S, Galla R, Molinari C, Uberti F. Design of Mixed Medicinal Plants, Rich in Polyphenols, Vitamins B, and Palmitoylethanolamide-Based Supplement to Help Reduce Nerve Pain: A Preclinical Study. International Journal of Molecular Sciences. 2024; 25(9):4790. https://doi.org/10.3390/ijms25094790
Chicago/Turabian StyleMulè, Simone, Giorgia Rosso, Mattia Botta, Arianna Brovero, Sara Ferrari, Rebecca Galla, Claudio Molinari, and Francesca Uberti. 2024. "Design of Mixed Medicinal Plants, Rich in Polyphenols, Vitamins B, and Palmitoylethanolamide-Based Supplement to Help Reduce Nerve Pain: A Preclinical Study" International Journal of Molecular Sciences 25, no. 9: 4790. https://doi.org/10.3390/ijms25094790