Studies on the Application of PGM Nanocatalysts from Spent Automotive Converters for Degradation of Ibuprofen in Aqueous Solutions
Abstract
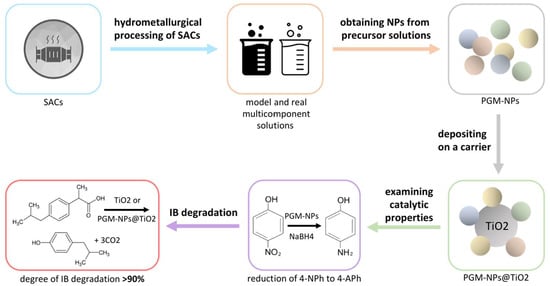
:1. Introduction
2. Results and Discussion
2.1. Catalysts from Model Solutions
2.2. Catalyst from a Real Solution
3. Materials and Methods
3.1. Reagents and Solutions
3.2. Synthesis of Nanoparticles
3.3. Catalytic Reaction
3.4. Apparatus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Kornak, N.; Kostecka, J. Elements of Social Perception of the Management of Expired Medicines. Polish J. Sustain. Dev. 2019, 23, 37–46. (In Polish) [Google Scholar] [CrossRef]
- Bavumiragira, J.P.; Ge, J.; Yin, H. Fate and Transport of Pharmaceuticals in Water Systems: A Processes Review. Sci. Total Environ. 2022, 823, 153635. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as Emerging Contaminants and Their Removal from Water: A Review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Varsha, M.; Senthil Kumar, P.; Senthil Rathi, B. A Review on Recent Trends in the Removal of Emerging Contaminants from Aquatic Environment Using Low-Cost Adsorbents. Chemosphere 2022, 287, 132270. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Pharmaceuticals in Drinking-Water; WHO: Paris, France, 2012. [Google Scholar]
- Sundararaman, S.; Aravind Kumar, J.; Deivasigamani, P.; Devarajan, Y. Emerging Pharma Residue Contaminants: Occurrence, Monitoring, Risk and Fate Assessment—A Challenge to Water Resource Management. Sci. Total Environ. 2022, 825, 153897. [Google Scholar] [CrossRef] [PubMed]
- Stachowicz, W.; Rzelewska-Piekut, M.; Regel-Rosocka, M. Should We Be Concerned about the Presence of Pharmaceuticals in Water? Technol. Wody 2023, 4, 36–43. (In Polish) [Google Scholar]
- Gunasekara, Y.; Kottawatta, S.; Nisansala, T.; Silva-Fletcher, A.; Kalupahana, R. Tackling Antimicrobial Resistance Needs One Health Approach. In One Health: Human, Animal, and Environment Triad; Wiley: Hoboken, NJ, USA, 2023; pp. 309–323. ISBN 9781119867333. [Google Scholar]
- The European Commission. Commission Implementing Decision (EU) 2022/1307 of 22 July 2022 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union 2022, L 197/117. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32022D1307 (accessed on 10 January 2024).
- Ravi, R.; Golder, A.K. A Tuneable Bioinspired Process of Pt-Doping in TiO2 for Improved Photoelectrochemical and Photocatalytic Functionalities. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 663, 131034. [Google Scholar] [CrossRef]
- Ziylan-Yavas, A.; Mizukoshi, Y.; Maeda, Y.; Ince, N.H. Supporting of Pristine TiO2 with Noble Metals to Enhance the Oxidation and Mineralization of Paracetamol by Sonolysis and Sonophotolysis. Appl. Catal. B Environ. 2015, 172–173, 7–17. [Google Scholar] [CrossRef]
- Ghaderi, S.; Lahafchi, R.T.; Jamshidi, S. Performance Evaluation of PdO/CuO TiO2 Photocatalytic Membrane on Ceramic Support for Removing Pharmaceutical Compounds from Water. J. Environ. Health Sci. Eng. 2023, 21, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; An, S.; Jho, E.H.; Bae, S.; Choi, Y.; Choe, J.K. Exploring Reductive Degradation of Fluorinated Pharmaceuticals Using Al2O3-Supported Pt-Group Metallic Catalysts: Catalytic Reactivity, Reaction Pathways, and Toxicity Assessment. Water Res. 2020, 185, 116242. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, N.; Sayadi, M.H.; Sobhani, S.; Hajiani, M. Photocatalytic Degradation of Model Pharmaceutical Pollutant by Novel Magnetic TiO2@ZnFe2O4/Pd Nanocomposite with Enhanced Photocatalytic Activity and Stability under Solar Light Irradiation. J. Environ. Manag. 2020, 271, 110964. [Google Scholar] [CrossRef] [PubMed]
- Thokchom, B.; Qiu, P.; Cui, M.; Park, B.; Pandit, A.B.; Khim, J. Magnetic Pd@Fe3O4 Composite Nanostructure as Recoverable Catalyst for Sonoelectrohybrid Degradation of Ibuprofen. Ultrason. Sonochem. 2017, 34, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, J.E. Greener Nanoscience: A Proactive Approach to Advancing Applications and Reducing Implications of Nanotechnology. ACS Nano 2008, 2, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Zan, G.; Wu, Q. Biomimetic and Bioinspired Synthesis of Nanomaterials/Nanostructures. Adv. Mater. 2016, 28, 2099–2147. [Google Scholar] [CrossRef] [PubMed]
- Rzelewska-Piekut, M.; Wolańczyk, Z.; Nowicki, M.; Regel-Rosocka, M. Precipitation of Pt, Pd, Rh, and Ru Nanoparticles with Non-Precious Metals from Model and Real Multicomponent Solutions. Molecules 2023, 28, 5188. [Google Scholar] [CrossRef]
- Wiecka, Z.; Cota, I.; Tylkowski, B.; Regel-Rosocka, M. Recovery of Platinum Group Metals from Spent Automotive Converters and Their Conversion into Efficient Recyclable Nanocatalysts. Environ. Sci. Pollut. Res. 2022, 30, 90168–90179. [Google Scholar] [CrossRef]
- Kezzim, A.; Boudjemaa, A.; Belhadi, A.; Trari, M. Photo-Catalytic Degradation of Ibuprofen over the New Semiconducting Catalyst α-(Cu,Fe)2O3 Prepared by Hydrothermal Route. Res. Chem. Intermed. 2017, 43, 3727–3743. [Google Scholar] [CrossRef]
- Padilla Villavicencio, M.; Escobedo Morales, A.; Ruiz Peralta, M.d.L.; Sánchez-Cantú, M.; Rojas Blanco, L.; Chigo Anota, E.; Camacho García, J.H.; Tzompantzi, F. Ibuprofen Photodegradation by Ag2O and Ag/Ag2O Composites Under Simulated Visible Light Irradiation. Catal. Lett. 2020, 150, 2385–2399. [Google Scholar] [CrossRef]
- Miranda, M.O.; Cabral Cavalcanti, W.E.; Barbosa, F.F.; de Sousa, J.A.; da Silva, F.I.; Pergher, S.B.C.; Braga, T.P. Photocatalytic Degradation of Ibuprofen Using Titanium Oxide: Insights into the Mechanism and Preferential Attack of Radicals. RSC Adv. 2021, 11, 27720–27733. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Romar, H.; Varila, T.; Xu, X.; Wang, Z.; Sillanpää, M.; Leiviskä, T. Ibuprofen Degradation Using a Co-Doped Carbon Matrix Derived from Peat as a Peroxymonosulphate Activator. Environ. Res. 2021, 193, 110564. [Google Scholar] [CrossRef]
- Tian, H.; Fan, Y.; Zhao, Y.; Liu, L. Elimination of Ibuprofen and Its Relative Photo-Induced Toxicity by Mesoporous BiOBr under Simulated Solar Light Irradiation. RSC Adv. 2014, 4, 13061. [Google Scholar] [CrossRef]
- Madhavan, J.; Grieser, F.; Ashokkumar, M. Combined Advanced Oxidation Processes for the Synergistic Degradation of Ibuprofen in Aqueous Environments. J. Hazard. Mater. 2010, 178, 202–208. [Google Scholar] [CrossRef]
- Jakimska, A.; Śliwka-Kaszyńska, M.; Reszczyńska, J.; Namieśnik, J.; Kot-Wasik, A. Elucidation of Transformation Pathway of Ketoprofen, Ibuprofen, and Furosemide in Surface Water and Their Occurrence in the Aqueous Environment Using UHPLC-QTOF-MS. Anal. Bioanal. Chem. 2014, 406, 3667–3680. [Google Scholar] [CrossRef]
- Marchlewicz, A.; Guzik, U.; Smułek, W.; Wojcieszyńska, D. Exploring the Degradation of Ibuprofen by Bacillus Thuringiensis B1(2015b): The New Pathway and Factors Affecting Degradation. Molecules 2017, 22, 1676. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Nurpeisova, D.T.; Barsbay, M. Effect of Copper Doping on the Photocatalytic Performance of Ni2O3@PC Membrane Composites in Norfloxacin Degradation. RSC Adv. 2024, 14, 4424–4435. [Google Scholar] [CrossRef]
- Tsvetkova, I.B.; Matveeva, V.G.; Doluda, V.Y.; Bykov, A.V.; Sidorov, A.I.; Schennikov, S.V.; Sulman, M.G.; Valetsky, P.M.; Stein, B.D.; Chen, C.-H.; et al. Pd(II) Nanoparticles in Porous Polystyrene: Factors Influencing the Nanoparticle Size and Catalytic Properties. J. Mater. Chem. 2012, 22, 6441. [Google Scholar] [CrossRef]
- Antolini, E. Structural Parameters of Supported Fuel Cell Catalysts: The Effect of Particle Size, Inter-Particle Distance and Metal Loading on Catalytic Activity and Fuel Cell Performance. Appl. Catal. B Environ. 2016, 181, 298–313. [Google Scholar] [CrossRef]
- Guettaıa, D.; Boudjemaa, A.; Zazoua, H.; Mokhtarı, M.; Bacharı, K. Effıcıent Photocatalytıc Degradatıon of the Pharmaceutical Ibuprofen over Fe-FSM-16 Photo-Catalyst under UV Irradıatıon. Int. J. Environ. Stud. 2021, 78, 444–458. [Google Scholar] [CrossRef]
- Ivanets, A.; Prozorovich, V.; Roshchina, M.; Grigoraviciute-Puroniene, I.; Zarkov, A.; Kareiva, A.; Wang, Z.; Srivastava, V.; Sillanpää, M. Heterogeneous Fenton Oxidation Using Magnesium Ferrite Nanoparticles for Ibuprofen Removal from Wastewater: Optimization and Kinetics Studies. J. Nanomater. 2020, 2020, 8159628. [Google Scholar] [CrossRef]
- Sun, S.-P.; Zeng, X.; Lemley, A.T. Nano-Magnetite Catalyzed Heterogeneous Fenton-like Degradation of Emerging Contaminants Carbamazepine and Ibuprofen in Aqueous Suspensions and Montmorillonite Clay Slurries at Neutral PH. J. Mol. Catal. A Chem. 2013, 371, 94–103. [Google Scholar] [CrossRef]
- Adityosulindro, S.; Julcour, C.; Barthe, L. Heterogeneous Fenton Oxidation Using Fe-ZSM5 Catalyst for Removal of Ibuprofen in Wastewater. J. Environ. Chem. Eng. 2018, 6, 5920–5928. [Google Scholar] [CrossRef]
- Saeid, S.; Kråkström, M.; Tolvanen, P.; Kumar, N.; Eränen, K.; Peurla, M.; Mikkola, J.-P.; Maël, L.; Kronberg, L.; Eklund, P.; et al. Synthesis and Characterization of Metal Modified Catalysts for Decomposition of Ibuprofen from Aqueous Solutions. Catalysts 2020, 10, 786. [Google Scholar] [CrossRef]
- Qin, M.; Jin, K.; Li, X.; Wang, R.; Li, Y.; Wang, H. Novel Highly-Active Ag/Bi Dual Nanoparticles-Decorated BiOBr Photocatalyst for Efficient Degradation of Ibuprofen. Environ. Res. 2022, 206, 112628. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Tang, C.; Oh, W.-D. Reinforced Degradation of Ibuprofen with MnCo2O4/FCNTs Nanocatalyst as Peroxymonosulfate Activator: Performance and Mechanism. J. Environ. Chem. Eng. 2022, 10, 107874. [Google Scholar] [CrossRef]
- da Silva, E.C.; de Moraes, M.O.S.; Brito, W.R.; Passos, R.R.; Brambilla, R.F.; da Costa, L.P.; Pocrifka, L.A. Synthesis of ZnO Nanoparticles by the Sol-Gel Protein Route: A Viable and Efficient Method for Photocatalytic Degradation of Methylene Blue and Ibuprofen. J. Bras. Chem. Soc. 2020, 31, 1648–1653. [Google Scholar] [CrossRef]
- Martins, M.; Mourato, C.; Sanches, S.; Noronha, J.P.; Crespo, M.T.B.; Pereira, I.A.C. Biogenic Platinum and Palladium Nanoparticles as New Catalysts for the Removal of Pharmaceutical Compounds. Water Res. 2017, 108, 160–168. [Google Scholar] [CrossRef] [PubMed]




| IB Degradation | Reaction pH | ||
|---|---|---|---|
| Without Catalyst | With TiO2 Alone | With Pd@TiO2 | |
| Before reaction | 4.7 | 4.7 | 4.7 |
| After 168 h | 4.9 | 5.2 | 5.7 |
| Reaction Time, min | IB Conversion, % | ||||
|---|---|---|---|---|---|
| Without Catalyst | TiO2 | Pd@TiO2 | Pt@TiO2 | Pt/Fe/Pd@TiO2 | |
| UV-Vis spectroscopy | |||||
| 5 | 22 | 34 | 35 | 70 | No data. |
| 15 | 38 | 46 | 50 | 74 | |
| 30 | 47 | 50 | 65 | 78 | |
| 60 | 50 | 63 | 76 | 80 | |
| 120 | 62 | 68 | 83 | 85 | |
| LC–MS/MS technique | |||||
| 5 | 40 | 40 | 52 | 50 | 88 |
| 15 | 52 | 69 | 53 | 65 | 91 |
| 30 | 87 | 77 | 63 | 82 | 92 |
| 60 | 88 | 84 | 70 | 91 | 92 |
| 120 | 93 | 90 | 91 | 95 | 94 |
| Reaction Time, min | k, min−1 | ||||
|---|---|---|---|---|---|
| Without a Catalyst | TiO2 | Pd@TiO2 | Pt@TiO2 | Pt/Fe/Pd@TiO2 | |
| 5 | 0.1015 | 0.1048 | 0.1486 | 0.1367 | 0.4140 |
| 15 | 0.0495 | 0.0779 | 0.0509 | 0.0682 | 0.1625 |
| 30 | 0.0690 | 0.0500 | 0.0332 | 0.0581 | 0.0852 |
| mean k | 0.0733 | 0.0776 | 0.0776 | 0.0877 | 0.2205 |
| Type of NP | Conditions of Degradation Reaction | IB Conversion | Ref. |
|---|---|---|---|
| Magnetic Pd@Fe3O4 | Enhanced by ultrasound and electrochemical reaction, 2 mg/dm3 IB, pH 5.2 | 100% in 60 min | [16] |
| Ag/Ag2O nanocomposite | Photoirradiation, 8 mg/dm3, room temperature, pH 3, 7, 9 | 30% | [22] |
| 15% | |||
| 17% | |||
| TiO2 | Photocatalysis 222 nm, 0.03 g TiO2, pH 5.0 | 100% in 5 min | [23] |
| Co-P 800/PMS | 10 mg/dm3 IB, 2 mM PMS, 0.1 g/dm3 catalyst, <23 °C, pH 7.6 | 90% in 120 min | [24] |
| BiOBr | Photocatalytic degradation—simulated solar light irradiation, 20 mg/dm3 IB, 0.5 g/dm3 BiOBr | 100% in 20 min | [25] |
| MgFe2O4 | Fenton-catalyst, 0.5 g/dm3 catalyst, 20 mmol/dm3 H2O2, 10 mg/dm3 IB, pH 6.0 | 100% in 40 min | [33] |
| Ag/Bi–BiOBr | Photocatalytic degradation—simulated solar light irradiation, 20 mg/dm3 IB, 30 mg/100 cm3 Ag/Bi–BiOBr | 92.3% in 60 min | [37] |
| MnCo2O4@FCNTs | Catalytic oxidation, 25.0 ± 0.2 °C, 10 mg/dm3 IB, pH 3–9 | >90% in 10 min | [38] |
| ZnO | UV light irradiation 254 nm, 25 °C, 20 mg/dm3 IB | 60% in 60 min | [39] |
| Biogenic Pt–NPs, Pd–NPs | Anaerobic conditions, 1 mg of the metallic cell NPs (25% of total mass composed by metal), 1 mg/dm3 IB, pH 5.3 | 0% | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolańczyk, Z.; Stachowicz, W.; Rzelewska-Piekut, M.; Zembrzuska, J.; Regel-Rosocka, M. Studies on the Application of PGM Nanocatalysts from Spent Automotive Converters for Degradation of Ibuprofen in Aqueous Solutions. Int. J. Mol. Sci. 2024, 25, 3147. https://doi.org/10.3390/ijms25063147
Wolańczyk Z, Stachowicz W, Rzelewska-Piekut M, Zembrzuska J, Regel-Rosocka M. Studies on the Application of PGM Nanocatalysts from Spent Automotive Converters for Degradation of Ibuprofen in Aqueous Solutions. International Journal of Molecular Sciences. 2024; 25(6):3147. https://doi.org/10.3390/ijms25063147
Chicago/Turabian StyleWolańczyk, Zuzanna, Wiktoria Stachowicz, Martyna Rzelewska-Piekut, Joanna Zembrzuska, and Magdalena Regel-Rosocka. 2024. "Studies on the Application of PGM Nanocatalysts from Spent Automotive Converters for Degradation of Ibuprofen in Aqueous Solutions" International Journal of Molecular Sciences 25, no. 6: 3147. https://doi.org/10.3390/ijms25063147