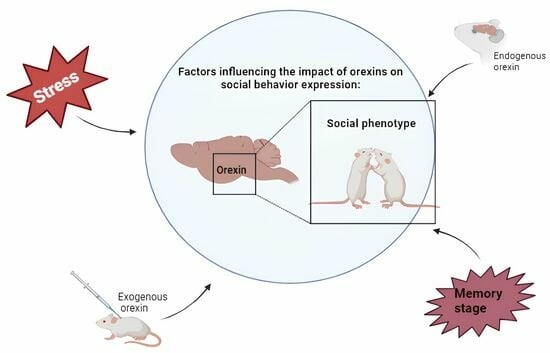
Orexins/Hypocretins: Gatekeepers of Social Interaction and Motivation
Abstract
:1. Introduction
2. Implication of Orexin in Social Behaviors upon Stress Exposure
3. Exogenous vs. Endogenous Orexins: Impact on SI
4. Involvement of Orexins in SI Depends on the Memory Phase
5. The Nucleus Accumbens Shell: A Pivotal Role in SI and Reward-Seeking Behavior via Orexins
6. How Orexin Impacts Social Motivation: Insight on a Potential Molecular Pathway
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, P.; Hong, W. Neural Circuit Mechanisms of Social Behavior. Neuron 2018, 98, 16–30. [Google Scholar] [CrossRef]
- El Rawas, R.; Saria, A. The Two Faces of Social Interaction Reward in Animal Models of Drug Dependence. Neurochem. Res. 2016, 41, 492–499. [Google Scholar] [CrossRef]
- Bardo, M.T.; Bevins, R.A. Conditioned Place Preference: What Does It Add to Our Preclinical Understanding of Drug Reward? Psychopharmacology 2000, 153, 31–43. [Google Scholar] [CrossRef]
- Tzschentke, T.M. Measuring Reward with the Conditioned Place Preference Paradigm: A Comprehensive Review of Drug Effects, Recent Progress and New Issues. Prog. Neurobiol. 1998, 56, 613–672. [Google Scholar] [CrossRef] [PubMed]
- Calcagnetti, D.J.; Schechter, M.D. Place Conditioning Reveals the Rewarding Aspect of Social Interaction in Juvenile Rats. Physiol. Behav. 1992, 51, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Douglas, L.A.; Varlinskaya, E.I.; Spear, L.P. Rewarding Properties of Social Interactions in Adolescent and Adult Male and Female Rats: Impact of Social versus Isolate Housing of Subjects and Partners. Dev. Psychobiol. 2004, 45, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.; El Rawas, R.; Salti, A.; Klement, S.; Bardo, M.T.; Kemmler, G.; Dechant, G.; Saria, A.; Zernig, G. Reversal of Cocaine-Conditioned Place Preference and Mesocorticolimbic Zif268 Expression by Social Interaction in Rats. Addict. Biol. 2011, 16, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, C.L.; Pijlman, F.T.; Koning, H.A.; Diergaarde, L.; Van Ree, J.M.; Spruijt, B.M. Isolation Changes the Incentive Value of Sucrose and Social Behaviour in Juvenile and Adult Rats. Behav. Brain Res. 1999, 106, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Yates, J.R.; Beckmann, J.S.; Meyer, A.C.; Bardo, M.T. Concurrent Choice for Social Interaction and Amphetamine Using Conditioned Place Preference in Rats: Effects of Age and Housing Condition. Drug Alcohol. Depend. 2013, 129, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Cremers, H.R.; Veer, I.M.; Spinhoven, P.; Rombouts, S.A.R.B.; Roelofs, K. Neural Sensitivity to Social Reward and Punishment Anticipation in Social Anxiety Disorder. Front. Behav. Neurosci. 2014, 8, 439. [Google Scholar] [CrossRef]
- Chevallier, C.; Kohls, G.; Troiani, V.; Brodkin, E.S.; Schultz, R.T. The Social Motivation Theory of Autism. Trends Cogn. Sci. 2012, 16, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Poisson, C.L.; Engel, L.; Saunders, B.T. Dopamine Circuit Mechanisms of Addiction-Like Behaviors. Front. Neural Circuits 2021, 15, 752420. [Google Scholar] [CrossRef] [PubMed]
- Rigney, N.; de Vries, G.J.; Petrulis, A.; Young, L.J. Oxytocin, Vasopressin, and Social Behavior: From Neural Circuits to Clinical Opportunities. Endocrinology 2022, 163, bqac111. [Google Scholar] [CrossRef] [PubMed]
- de Lecea, L.; Kilduff, T.S.; Peyron, C.; Gao, X.; Foye, P.E.; Danielson, P.E.; Fukuhara, C.; Battenberg, E.L.; Gautvik, V.T.; Bartlett, F.S.; et al. The Hypocretins: Hypothalamus-Specific Peptides with Neuroexcitatory Activity. Proc. Natl. Acad. Sci. USA 1998, 95, 322–327. [Google Scholar] [CrossRef]
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors That Regulate Feeding Behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef]
- Jöhren, O.; Neidert, S.J.; Kummer, M.; Dendorfer, A.; Dominiak, P. Prepro-Orexin and Orexin Receptor mRNAs Are Differentially Expressed in Peripheral Tissues of Male and Female Rats. Endocrinology 2001, 142, 3324–3331. [Google Scholar] [CrossRef]
- Mieda, M.; Tsujino, N.; Sakurai, T. Differential Roles of Orexin Receptors in the Regulation of Sleep/Wakefulness. Front. Endocrinol. 2013, 4, 57. [Google Scholar] [CrossRef] [PubMed]
- Bourgin, P.; Huitrón-Reséndiz, S.; Spier, A.D.; Fabre, V.; Morte, B.; Criado, J.R.; Sutcliffe, J.G.; Henriksen, S.J.; de Lecea, L. Hypocretin-1 Modulates Rapid Eye Movement Sleep through Activation of Locus Coeruleus Neurons. J. Neurosci. 2000, 20, 7760–7765. [Google Scholar] [CrossRef]
- Hagan, J.J.; Leslie, R.A.; Patel, S.; Evans, M.L.; Wattam, T.A.; Holmes, S.; Benham, C.D.; Taylor, S.G.; Routledge, C.; Hemmati, P.; et al. Orexin A Activates Locus Coeruleus Cell Firing and Increases Arousal in the Rat. Proc. Natl. Acad. Sci. USA 1999, 96, 10911–10916. [Google Scholar] [CrossRef]
- Prober, D.A.; Rihel, J.; Onah, A.A.; Sung, R.-J.; Schier, A.F. Hypocretin/Orexin Overexpression Induces An Insomnia-Like Phenotype in Zebrafish. J. Neurosci. 2006, 26, 13400–13410. [Google Scholar] [CrossRef]
- Tang, S.; Huang, W.; Lu, S.; Lu, L.; Li, G.; Chen, X.; Liu, X.; Lv, X.; Zhao, Z.; Duan, R.; et al. Increased Plasma Orexin-A Levels in Patients with Insomnia Disorder Are Not Associated with Prepro-Orexin or Orexin Receptor Gene Polymorphisms. Peptides 2017, 88, 55–61. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.C.; do Carmo, A.S.; dos Santos, L.C. Associations between Sleep Practices and Social Behavior of Children and Adolescents: A Systematic Review. J. Public. Health 2022, 30, 1101–1112. [Google Scholar] [CrossRef]
- Samson, W.K.; Gosnell, B.; Chang, J.K.; Resch, Z.T.; Murphy, T.C. Cardiovascular Regulatory Actions of the Hypocretins in Brain. Brain Res. 1999, 831, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Kayaba, Y.; Nakamura, A.; Kasuya, Y.; Ohuchi, T.; Yanagisawa, M.; Komuro, I.; Fukuda, Y.; Kuwaki, T. Attenuated Defense Response and Low Basal Blood Pressure in Orexin Knockout Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R581–R593. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Tang, H.; Liu, Y.; Yuan, Y.; Wang, M. Research Progress on the Mechanism of Orexin in Pain Regulation in Different Brain Regions. Open Life Sci. 2021, 16, 46–52. [Google Scholar] [CrossRef]
- Baldo, B.A.; Daniel, R.A.; Berridge, C.W.; Kelley, A.E. Overlapping Distributions of Orexin/Hypocretin- and Dopamine-β-Hydroxylase Immunoreactive Fibers in Rat Brain Regions Mediating Arousal, Motivation, and Stress. J. Comp. Neurol. 2003, 464, 220–237. [Google Scholar] [CrossRef]
- DiLeone, R.J.; Georgescu, D.; Nestler, E.J. Lateral Hypothalamic Neuropeptides in Reward and Drug Addiction. Life Sci. 2003, 73, 759–768. [Google Scholar] [CrossRef]
- Katzman, M.A.; Katzman, M.P. Neurobiology of the Orexin System and Its Potential Role in the Regulation of Hedonic Tone. Brain Sci. 2022, 12, 150. [Google Scholar] [CrossRef]
- Baimel, C.; Bartlett, S.E.; Chiou, L.-C.; Lawrence, A.J.; Muschamp, J.W.; Patkar, O.; Tung, L.-W.; Borgland, S.L. Orexin/Hypocretin Role in Reward: Implications for Opioid and Other Addictions. Br. J. Pharmacol. 2015, 172, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Couvineau, A.; Voisin, T.; Nicole, P.; Gratio, V.; Abad, C.; Tan, Y.-V. Orexins as Novel Therapeutic Targets in Inflammatory and Neurodegenerative Diseases. Front. Endocrinol. 2019, 10, 709. [Google Scholar] [CrossRef] [PubMed]
- Faustmann, T.J.; Kamp, D.; Räuber, S.; Dukart, J.; Melzer, N.; Schilbach, L. Social Interaction, Psychotic Disorders and Inflammation: A Triangle of Interest. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 122, 110697. [Google Scholar] [CrossRef] [PubMed]
- Faesel, N.; Kolodziejczyk, M.H.; Koch, M.; Fendt, M. Orexin Deficiency Affects Sociability and the Acquisition, Expression, and Extinction of Conditioned Social Fear. Brain Res. 2021, 1751, 147199. [Google Scholar] [CrossRef]
- Abbas, M.G.; Shoji, H.; Soya, S.; Hondo, M.; Miyakawa, T.; Sakurai, T. Comprehensive Behavioral Analysis of Male Ox1r (-/-) Mice Showed Implication of Orexin Receptor-1 in Mood, Anxiety, and Social Behavior. Front. Behav. Neurosci. 2015, 9, 324. [Google Scholar] [CrossRef]
- Yang, L.; Zou, B.; Xiong, X.; Pascual, C.; Xie, J.; Malik, A.; Xie, J.; Sakurai, T.; Xie, X.S. Hypocretin/Orexin Neurons Contribute to Hippocampus-Dependent Social Memory and Synaptic Plasticity in Mice. J. Neurosci. 2013, 33, 5275–5284. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.; Terstege, D.J.; Jamani, N.; Tsutsui, M.; Pavlov, D.; Bugescu, R.; Epp, J.R.; Leinninger, G.M.; Sargin, D. Hypocretin/Orexin Neurons Encode Social Discrimination and Exhibit a Sex-Dependent Necessity for Social Interaction. Cell Rep. 2023, 42, 112815. [Google Scholar] [CrossRef]
- Blouin, A.M.; Fried, I.; Wilson, C.L.; Staba, R.J.; Behnke, E.J.; Lam, H.A.; Maidment, N.T.; Karlsson, K.Æ.; Lapierre, J.L.; Siegel, J.M. Human Hypocretin and Melanin-Concentrating Hormone Levels Are Linked to Emotion and Social Interaction. Nat. Commun. 2013, 4, 1547. [Google Scholar] [CrossRef] [PubMed]
- Granza, A.E.; Amaral, I.M.; Monteiro, D.G.; Salti, A.; Hofer, A.; El Rawas, R. Social Interaction Is Less Rewarding in Adult Female than in Male Mice. Brain Sci. 2023, 13, 1445. [Google Scholar] [CrossRef] [PubMed]
- Reppucci, C.J.; Gergely, C.K.; Bredewold, R.; Veenema, A.H. Involvement of Orexin/Hypocretin in the Expression of Social Play Behaviour in Juvenile Rats. Int. J. Play. 2020, 9, 108–127. [Google Scholar] [CrossRef]
- Berridge, C.W.; España, R.A. Hypocretins: Waking, Arousal, or Action? Neuron 2005, 46, 696–698. [Google Scholar] [CrossRef]
- Furlong, T.M.; Vianna, D.M.L.; Liu, L.; Carrive, P. Hypocretin/Orexin Contributes to the Expression of Some but Not All Forms of Stress and Arousal. Eur. J. Neurosci. 2009, 30, 1603–1614. [Google Scholar] [CrossRef]
- Steiner, M.A.; Sciarretta, C.; Brisbare-Roch, C.; Strasser, D.S.; Studer, R.; Jenck, F. Examining the Role of Endogenous Orexins in Hypothalamus-Pituitary-Adrenal Axis Endocrine Function Using Transient Dual Orexin Receptor Antagonism in the Rat. Psychoneuroendocrinology 2013, 38, 560–571. [Google Scholar] [CrossRef]
- Al-Barazanji, K.A.; Wilson, S.; Baker, J.; Jessop, D.S.; Harbuz, M.S. Central Orexin-A Activates Hypothalamic-Pituitary-Adrenal Axis and Stimulates Hypothalamic Corticotropin Releasing Factor and Arginine Vasopressin Neurones in Conscious Rats. J. Neuroendocrinol. 2001, 13, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Kuru, M.; Ueta, Y.; Serino, R.; Nakazato, M.; Yamamoto, Y.; Shibuya, I.; Yamashita, H. Centrally Administered Orexin/Hypocretin Activates HPA Axis in Rats. Neuroreport 2000, 11, 1977–1980. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.H.; Small, C.J.; Dakin, C.L.; Abbott, C.R.; Morgan, D.G.; Ghatei, M.A.; Bloom, S.R. The Central Effects of Orexin-A in the Hypothalamic-Pituitary-Adrenal Axis in Vivo and in Vitro in Male Rats. J. Neuroendocrinol. 2001, 13, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G.; Smith, R.J.; Moorman, D.E.; Richardson, K.A. Role of Lateral Hypothalamic Orexin Neurons in Reward Processing and Addiction. Neuropharmacology 2009, 56 (Suppl. 1), 112–121. [Google Scholar] [CrossRef] [PubMed]
- Grafe, L.A.; Bhatnagar, S. Orexins and Stress. Front. Neuroendocrinol. 2018, 51, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Eacret, D.; Grafe, L.A.; Dobkin, J.; Gotter, A.L.; Renger, J.J.; Winrow, C.J.; Bhatnagar, S. Orexin Signaling during Social Defeat Stress Influences Subsequent Social Interaction Behaviour and Recognition Memory. Behav. Brain Res. 2019, 356, 444–452. [Google Scholar] [CrossRef]
- Grafe, L.A.; Eacret, D.; Dobkin, J.; Bhatnagar, S. Reduced Orexin System Function Contributes to Resilience to Repeated Social Stress. eNeuro 2018, 5, ENEURO.0273-17.2018. [Google Scholar] [CrossRef]
- Heydendael, W.; Sengupta, A.; Beck, S.; Bhatnagar, S. Optogenetic Examination Identifies a Context-Specific Role for Orexins/Hypocretins in Anxiety-Related Behavior. Physiol. Behav. 2014, 130, 182–190. [Google Scholar] [CrossRef]
- Jöhren, O.; Brüggemann, N.; Dendorfer, A.; Dominiak, P. Gonadal Steroids Differentially Regulate the Messenger Ribonucleic Acid Expression of Pituitary Orexin Type 1 Receptors and Adrenal Orexin Type 2 Receptors. Endocrinology 2003, 144, 1219–1225. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Shwani, T.; Liu, J.; Zhong, P.; Yang, F.; Schatz, K.; Zhang, F.; Pralle, A.; Yan, Z. Molecular and Cellular Mechanisms for Differential Effects of Chronic Social Isolation Stress in Males and Females. Mol. Psychiatry 2022, 27, 3056–3068. [Google Scholar] [CrossRef]
- Luo, F.; Deng, J.-Y.; Sun, X.; Zhen, J.; Luo, X.-D. Anterior Cingulate Cortex Orexin Signaling Mediates Early-Life Stress-Induced Social Impairment in Females. Proc. Natl. Acad. Sci. USA 2023, 120, e2220353120. [Google Scholar] [CrossRef]
- Barretto-de-Souza, L.; Joseph, S.A.; Lynch, F.M.; Ng, A.J.; Crestani, C.C.; Christianson, J.P. Melanin-Concentrating Hormone and Orexin Shape Social Affective Behavior via Action in the Insular Cortex of Rat. Psychopharmacology 2023. [Google Scholar] [CrossRef]
- Ji, M.-J.; Zhang, X.-Y.; Chen, Z.; Wang, J.-J.; Zhu, J.-N. Orexin Prevents Depressive-like Behavior by Promoting Stress Resilience. Mol. Psychiatry 2019, 24, 282–293. [Google Scholar] [CrossRef]
- Hebb, D.O. The Organization of Behavior: A Neuropsychological Theory; Wiley: Oxford, UK, 1949; pp. xix, 335. [Google Scholar]
- Mazur, F.; Całka, J. Hypothalamic Orexins as Possible Therapeutic Agents in Threat and Spatial Memory Disorders. Front. Behav. Neurosci. 2023, 17, 1228056. [Google Scholar] [CrossRef]
- Roozendaal, B. Stress and Memory: Opposing Effects of Glucocorticoids on Memory Consolidation and Memory Retrieval. Neurobiol. Learn. Mem. 2002, 78, 578–595. [Google Scholar] [CrossRef]
- Roozendaal, B.; Okuda, S.; Van der Zee, E.A.; McGaugh, J.L. Glucocorticoid Enhancement of Memory Requires Arousal-Induced Noradrenergic Activation in the Basolateral Amygdala. Proc. Natl. Acad. Sci. USA 2006, 103, 6741–6746. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, R.; Fornari, R.V.; Roozendaal, B. Glucocorticoids Interact with the Noradrenergic Arousal System in the Nucleus Accumbens Shell to Enhance Memory Consolidation of Both Appetitive and Aversive Taste Learning. Neurobiol. Learn. Mem. 2012, 98, 197–205. [Google Scholar] [CrossRef]
- Eriksson, K.S.; Sergeeva, O.A.; Haas, H.L.; Selbach, O. Orexins/Hypocretins and Aminergic Systems. Acta Physiol. 2010, 198, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Carlezon, W.A. The Mesolimbic Dopamine Reward Circuit in Depression. Biol. Psychiatry 2006, 59, 1151–1159. [Google Scholar] [CrossRef]
- Meredith, G.E.; Agolia, R.; Arts, M.P.; Groenewegen, H.J.; Zahm, D.S. Morphological Differences between Projection Neurons of the Core and Shell in the Nucleus Accumbens of the Rat. Neuroscience 1992, 50, 149–162. [Google Scholar] [CrossRef]
- Meredith, G.E.; Pennartz, C.M.; Groenewegen, H.J. The Cellular Framework for Chemical Signalling in the Nucleus Accumbens. Prog. Brain Res. 1993, 99, 3–24. [Google Scholar] [CrossRef]
- Peyron, C.; Tighe, D.K.; van den Pol, A.N.; de Lecea, L.; Heller, H.C.; Sutcliffe, J.G.; Kilduff, T.S. Neurons Containing Hypocretin (Orexin) Project to Multiple Neuronal Systems. J. Neurosci. 1998, 18, 9996–10015. [Google Scholar] [CrossRef] [PubMed]
- Amaral, I.M.; Scheffauer, L.; Hofer, A.; El Rawas, R. Protein Kinases in Natural versus Drug Reward. Pharmacol. Biochem. Behav. 2022, 221, 173472. [Google Scholar] [CrossRef]
- Amaral, I.M.; Scheffauer, L.; Langeder, A.B.; Hofer, A.; El Rawas, R. Rewarding Social Interaction in Rats Increases CaMKII in the Nucleus Accumbens. Biomedicines 2021, 9, 1886. [Google Scholar] [CrossRef] [PubMed]
- Salti, A.; Kummer, K.K.; Sadangi, C.; Dechant, G.; Saria, A.; El Rawas, R. Social Interaction Reward Decreases P38 Activation in the Nucleus Accumbens Shell of Rats. Neuropharmacology 2015, 99, 510–516. [Google Scholar] [CrossRef]
- Lemos, C.; Salti, A.; Amaral, I.M.; Fontebasso, V.; Singewald, N.; Dechant, G.; Hofer, A.; El Rawas, R. Social Interaction Reward in Rats Has Anti-stress Effects. Addict. Biol. 2021, 26, e12878. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.; El Rawas, R.; Klement, S.; Kummer, K.; Mayr, M.J.; Eggart, V.; Salti, A.; Bardo, M.T.; Saria, A.; Zernig, G. Differential Effects of Accumbens Core vs. Shell Lesions in a Rat Concurrent Conditioned Place Preference Paradigm for Cocaine vs. Social Interaction. PLoS ONE 2011, 6, e26761. [Google Scholar] [CrossRef]
- Olaniran, A.; Garcia, K.T.; Burke, M.A.M.; Lin, H.; Venniro, M.; Li, X. Operant Social Seeking to a Novel Peer after Social Isolation Is Associated with Activation of Nucleus Accumbens Shell in Rats. Psychopharmacology 2022, ahead of print. [Google Scholar] [CrossRef]
- Mori, K.; Kim, J.; Sasaki, K. Electrophysiological Effects of Orexin-B and Dopamine on Rat Nucleus Accumbens Shell Neurons In Vitro. Peptides 2011, 32, 246–252. [Google Scholar] [CrossRef]
- Lei, K.; Wegner, S.A.; Yu, J.H.; Mototake, A.; Hu, B.; Hopf, F.W. Nucleus Accumbens Shell and mPFC but Not Insula Orexin-1 Receptors Promote Excessive Alcohol Drinking. Front. Neurosci. 2016, 10, 400. [Google Scholar] [CrossRef]
- Patyal, R.; Woo, E.Y.; Borgland, S.L. Local Hypocretin-1 Modulates Terminal Dopamine Concentration in the Nucleus Accumbens Shell. Front. Behav. Neurosci. 2012, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Wei, C.; Li, Y.; Sui, N. Orexin Receptors within the Nucleus Accumbens Shell Mediate the Stress but Not Drug Priming-Induced Reinstatement of Morphine Conditioned Place Preference. Front. Behav. Neurosci. 2013, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Miura, S.; Yoshida, T.; Kim, J.; Sasaki, K. Cytosolic Calcium Elevation Induced by Orexin/Hypocretin in Granule Cell Domain Cells of the Rat Cochlear Nucleus in Vitro. Peptides 2010, 31, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.; Ekholm, M.E.; Kukkonen, J.P. Multiple Phospholipase Activation by OX1 Orexin/Hypocretin Receptors. Cell. Mol. Life Sci. 2008, 65, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Q.; Ji, B.; Pan, Y.; Xu, C.; Cheng, B.; Bai, B.; Chen, J. The Orexin/Receptor System: Molecular Mechanism and Therapeutic Potential for Neurological Diseases. Front. Mol. Neurosci. 2018, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Monda, V.; Sessa, F.; Valenzano, A.; Salerno, M.; Bitetti, I.; Precenzano, F.; Marotta, R.; Lavano, F.; Lavano, S.M.; et al. Sympathetic, Metabolic Adaptations, and Oxidative Stress in Autism Spectrum Disorders: How Far From Physiology? Front. Physiol. 2018, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Dichter, G.S.; Damiano, C.A.; Allen, J.A. Reward Circuitry Dysfunction in Psychiatric and Neurodevelopmental Disorders and Genetic Syndromes: Animal Models and Clinical Findings. J. Neurodev. Disord. 2012, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Zametkin, A.J.; Matochik, J.A.; Pascualvaca, D.; Cohen, R.M. Low Medial Prefrontal Dopaminergic Activity in Autistic Children. Lancet 1997, 350, 638. [Google Scholar] [CrossRef]
- Scott-Van Zeeland, A.A.; Dapretto, M.; Ghahremani, D.G.; Poldrack, R.A.; Bookheimer, S.Y. Reward Processing in Autism. Autism Res. 2010, 3, 53–67. [Google Scholar] [CrossRef]
- Calipari, E.S.; España, R.A. Hypocretin/Orexin Regulation of Dopamine Signaling: Implications for Reward and Reinforcement Mechanisms. Front. Behav. Neurosci. 2012, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Vittoz, N.M.; Berridge, C.W. Hypocretin/Orexin Selectively Increases Dopamine Efflux within the Prefrontal Cortex: Involvement of the Ventral Tegmental Area. Neuropsychopharmacology 2006, 31, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Shi, Y.; Chan, M.T.V.; Peng, G.; Zhang, Q.; Sun, X.; Zhu, Z.; Xie, Y.; Sham, K.W.Y.; Li, J.; et al. Developmental Protein Kinase C Hyper-Activation Results in Microcephaly and Behavioral Abnormalities in Zebrafish. Transl. Psychiatry 2018, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Namkung, Y.; Sibley, D.R. Protein Kinase C Mediates Phosphorylation, Desensitization, and Trafficking of the D2 Dopamine Receptor. J. Biol. Chem. 2004, 279, 49533–49541. [Google Scholar] [CrossRef]
- Gingrich, B.; Liu, Y.; Cascio, C.; Wang, Z.; Insel, T.R. Dopamine D2 Receptors in the Nucleus Accumbens Are Important for Social Attachment in Female Prairie Voles (Microtus ochrogaster). Behav. Neurosci. 2000, 114, 173–183. [Google Scholar] [CrossRef]
- Trifilieff, P.; Feng, B.; Urizar, E.; Winiger, V.; Ward, R.D.; Taylor, K.M.; Martinez, D.M.; Moore, H.; Balsam, P.D.; Simpson, E.H.; et al. Increasing Dopamine D2 Receptor Expression in the Adult Nucleus Accumbens Enhances Motivation. Mol. Psychiatry 2013, 18, 1025–1033. [Google Scholar] [CrossRef]
- Zhang, X.; Xun, Y.; Wang, L.; Zhang, J.; Hou, W.; Ma, H.; Cai, W.; Li, L.; Guo, Q.; Li, Y.; et al. Involvement of the Dopamine System in the Effect of Chronic Social Isolation during Adolescence on Social Behaviors in Male C57 Mice. Brain Res. 2021, 1765, 147497. [Google Scholar] [CrossRef]
- Berridge, K.C.; Robinson, T.E. What Is the Role of Dopamine in Reward: Hedonic Impact, Reward Learning, or Incentive Salience? Brain Res. Brain Res. Rev. 1998, 28, 309–369. [Google Scholar] [CrossRef]
- Salamone, J.D.; Correa, M.; Farrar, A.; Mingote, S.M. Effort-Related Functions of Nucleus Accumbens Dopamine and Associated Forebrain Circuits. Psychopharmacology 2007, 191, 461–482. [Google Scholar] [CrossRef]



| Study | Molecular | Relation | Stress | Results in | Reference |
|---|---|---|---|---|---|
| Orexin signaling during social defeat stress influences subsequent SI behavior and recognition memory | Orexin stimulation, decreased SI | Inverse | + | Males | [47] |
| Reduced orexin system function contributes to resilience to repeated social stress | Orexin inhibition, increased SI Low prepro-orexin mRNA in resilient rats | Inverse | + | Males | [48] |
| Optogenetic examination identifies a context-specific role for orexins/hypocretins in anxiety-related behavior | Orexin photostimulation, reduced SI time | Inverse | + | Males | [49] |
| Involvement of orexin/hypocretin in the expression of social play behavior in juvenile rats | OXA administration, decreased social play | Inverse | − | Males, females | [38] |
| Orexin deficiency affects sociability and the acquisition, expression, and extinction of conditioned social fear | Orexin deficiency, reduced sociability and preference for social novelty in female mice | Direct | − | Females | [32] |
| Comprehensive behavioral analysis of male Ox1r (−/−) mice showed implication of orexin receptor-1 in mood, anxiety, and social behavior | OX1R−/− mice, decreased SI, increased anxiety-like behavior | Direct | − | Males | [33] |
| Hypocretin/orexin neurons encode social discrimination and exhibit a sex-dependent necessity for social interaction | OX1R blockade, reduced SI in male, but not female mice | Direct | − | Males | [35] |
| Molecular and cellular mechanisms for differential effects of chronic social isolation stress in males and females | OXA treatment in female stressed mice, social withdrawal rescued | Direct | + | Females | [51] |
| Anterior cingulate cortex orexin-signaling mediates early-life stress-induced social impairment in females | ACC orexin terminal activation, diminished sociability rescued | Direct | + | Females | [52] |
| Hypocretin/orexin neurons contribute to hippocampus-dependent social memory and synaptic plasticity in mice | OXA nasal administration, social memory restored | Direct | − | Males, females | [34] |
| Human hypocretin and melanin concentrating hormone levels are linked to emotion and SI | OXA levels maximal during SI, positive emotions and anger | Direct | − | − | [36] |
| Orexin’s Source | Mode of Action | References | Conclusion |
|---|---|---|---|
| Exogenous | OXA administration, reduced social play time and allogrooming behaviors | [38] | |
| OXA microinjection, increased interaction time in one-on-one SI test | [53] | Exogenous sources are capable of the alteration of social behavior expression | |
| OXA bilateral microinjection, increase in the time spent in exploring the area close to a caged novel rat and the number of times climbing this cage | [54] | ||
| Endogenous | OX1R knockdown, no effect on novel SI In case of stress, alleviation of social avoidation by endogenous orexin | [54] | The impact of endogenous orexins on SI varies depending on certain conditions, namely: stress, sex of the investigated species, methods used for social behavior assessment, etc. |
| Decreased sociability and preference for social novelty observed only in female, but not male orexin-deficient mice | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouaidat, S.; Amaral, I.M.; Monteiro, D.G.; Harati, H.; Hofer, A.; El Rawas, R. Orexins/Hypocretins: Gatekeepers of Social Interaction and Motivation. Int. J. Mol. Sci. 2024, 25, 2609. https://doi.org/10.3390/ijms25052609
Ouaidat S, Amaral IM, Monteiro DG, Harati H, Hofer A, El Rawas R. Orexins/Hypocretins: Gatekeepers of Social Interaction and Motivation. International Journal of Molecular Sciences. 2024; 25(5):2609. https://doi.org/10.3390/ijms25052609
Chicago/Turabian StyleOuaidat, Sara, Inês M. Amaral, Diogo G. Monteiro, Hayat Harati, Alex Hofer, and Rana El Rawas. 2024. "Orexins/Hypocretins: Gatekeepers of Social Interaction and Motivation" International Journal of Molecular Sciences 25, no. 5: 2609. https://doi.org/10.3390/ijms25052609