Molecular-Scale Investigations Reveal the Effect of Natural Polyphenols on BAX/Bcl-2 Interactions
Abstract
:1. Introduction
2. Results and Discussion
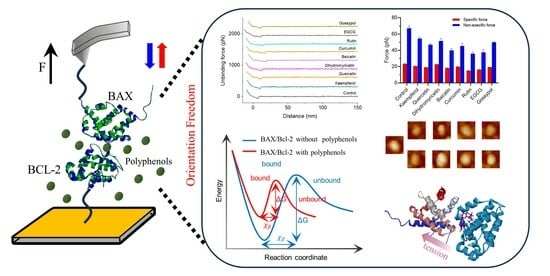
2.1. Probing the Inhibition of BAX/Bcl-2 Binding by Polyphenols Using AFM
2.2. Probing Single-Molecule Interaction Forces between BAX and Bcl-2 in Polyphenols
2.3. Probing the Kinetic Properties of Polyphenol-Regulated BAX/Bcl-2 Dissociation
2.4. Probing the Kinetic Properties of Polyphenol-Regulated BAX/Bcl-2 Association
2.5. Exploring the Polyphenols Modulating BAX/Bcl-2 Interfacial Properties
2.6. Morphological Changes of Bcl-2 Protein
2.7. Polyphenol Binding to Bcl-2 Competes for BAX
3. Materials and Methods
3.1. Materials and Reagents
3.2. Immobilization of Proteins on AFM Tips and Substrate
3.3. Single-Molecule Force Spectroscopy Measurements between BAX an Bcl-2 on Model Substrates
3.4. Morphological Changes in Protein
3.5. Theoretical Model Used to Quantitatively Analyze the Interactions
3.6. Interfacial Property Measurements Using Contact Angle
3.7. Molecular Docking
3.8. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Westphal, D.; Kluck, R.M.; Dewson, G. Building blocks of the apoptotic pore: How Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ. 2014, 21, 196–205. [Google Scholar] [CrossRef]
- Dlugosz, P.J.; Billen, L.P.; Annis, M.G.; Zhu, W.; Zhang, Z.; Lin, J.; Leber, B.; Andrews, D.W. Bcl-2 changes conformation to inhibit Bax oligomerization. EMBO J. 2006, 25, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Renault, T.T.; Floros, K.V.; Chipuk, J.E. BAK/BAX activation and cytochrome c release assays using isolated mitochondria. Methods 2013, 61, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.Y.; Lin, L.T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 2015, 35, S78–S103. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Roberts, A.W.; Colman, P.M.; Adams, J.M. Targeting BCL-2-like Proteins to Kill Cancer Cells. Trends Cancer 2016, 2, 443–460. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, S.; Rahmani, M. Targeting BCL-2 in Cancer: Advances, Challenges, and Perspectives. Cancers 2021, 13, 1292. [Google Scholar] [CrossRef] [PubMed]
- Birkinshaw, R.W.; Gong, J.-N.; Luo, C.S.; Lio, D.; White, C.A.; Anderson, M.A.; Blombery, P.; Lessene, G.; Majewski, I.J.; Thijssen, R.; et al. Structures of BCL-2 in complex with venetoclax reveal the molecular basis of resistance mutations. Nat. Commun. 2019, 10, 2385. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Z.; Zhao, X. Targeting Bcl-2 for cancer therapy. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188569. [Google Scholar] [CrossRef]
- Shin, W.H.; Kumazawa, K.; Imai, K.; Hirokawa, T.; Kihara, D. Current Challenges and Opportunities in Designing Protein-Protein Interaction Targeted Drugs. Adv. Appl. Bioinform. Chem. 2020, 13, 11–25. [Google Scholar] [CrossRef]
- Truong, J.; George, A.; Holien, J.K. Analysis of physicochemical properties of protein-protein interaction modulators suggests stronger alignment with the “rule of five”. RSC Med. Chem. 2021, 12, 1731–1749. [Google Scholar] [CrossRef]
- Guo, Q.; Cao, H.; Qi, X.; Li, H.; Ye, P.; Wang, Z.; Wang, D.; Sun, M. Research Progress in Reversal of Tumor Multi-Drug Resistance via Natural Products. Anticancer Agents Med. Chem. 2017, 17, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Phosrithong, N.; Ungwitayatorn, J. Molecular docking study on anticancer activity of plant-derived natural products. Med. Chem. Res. 2009, 19, 817–835. [Google Scholar] [CrossRef]
- Papademetrio, D.L.; Trabucchi, A.; Cavaliere, V.; Ricco, R.; Costantino, S.; Wagner, M.L.; Álvarez, E. The catechin flavonoid reduces proliferation and induces apoptosis of murine lymphoma cells LB02 through modulation of antiapoptotic proteins. Rev. Bras. Farmacogn. 2013, 23, 455–463. [Google Scholar] [CrossRef]
- Primikyri, A.; Chatziathanasiadou, M.V.; Karali, E.; Kostaras, E.; Mantzaris, M.D.; Hatzimichael, E.; Shin, J.S.; Chi, S.W.; Briasoulis, E.; Kolettas, E.; et al. Direct binding of Bcl-2 family proteins by quercetin triggers its pro-apoptotic activity. ACS Chem. Biol. 2014, 9, 2737–2741. [Google Scholar] [CrossRef]
- Saraei, R.; Marofi, F.; Naimi, A.; Talebi, M.; Ghaebi, M.; Javan, N.; Salimi, O.; Hassanzadeh, A. Leukemia therapy by flavonoids: Future and involved mechanisms. J. Cell. Physiol. 2019, 234, 8203–8220. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Khan, H.; Zia, A.; Khan, A.; Fakhri, S.; Aschner, M.; Gul, K.; Saso, L. Bcl-2 Modulation in p53 Signaling Pathway by Flavonoids: A Potential Strategy towards the Treatment of Cancer. Int. J. Mol. Sci. 2021, 22, 11315. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Kamarudin, M.N.A.; Naidu, R. Anticancer Mechanism of Flavonoids on High-Grade Adult-Type Diffuse Gliomas. Nutrients 2023, 15, 797. [Google Scholar] [CrossRef]
- Muller, D.J.; Dumitru, A.C.; Lo Giudice, C.; Gaub, H.E.; Hinterdorfer, P.; Hummer, G.; De Yoreo, J.J.; Dufrene, Y.F.; Alsteens, D. Atomic Force Microscopy-Based Force Spectroscopy and Multiparametric Imaging of Biomolecular and Cellular Systems. Chem. Rev. 2021, 121, 11701–11725. [Google Scholar] [CrossRef]
- Lostao, A.; Lim, K.; Pallares, M.C.; Ptak, A.; Marcuello, C. Recent advances in sensing the inter-biomolecular interactions at the nanoscale—A comprehensive review of AFM-based force spectroscopy. Int. J. Biol. Macromol. 2023, 238, 124089. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, B.; Sun, Z.; Esfahani, A.M.; Hou, J.; Jiao, N.D.; Liu, L.; Chen, L.; Basson, M.D.; Dong, L.; et al. Optimization of Protein-Protein Interaction Measurements for Drug Discovery Using AFM Force Spectroscopy. IEEE Trans. Nanotechnol. 2019, 18, 509–517. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J. Novel perspective for protein-drug interaction analysis: Atomic force microscope. Analyst 2023, 148, 454–474. [Google Scholar] [CrossRef]
- Sun, H.; Tian, Y.; Fu, Y.; Lei, Y.; Wang, Y.; Yan, X.; Wang, J. Single-molecule scale quantification reveals interactions underlying protein-protein interface: From forces to non-covalent bonds. Phys. Chem. Chem. Phys. 2023, 25, 31791–31803. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, W.; Xu, Z.; Zhang, X.F.; Liu, Y. Binding kinetics of liposome conjugated E-selectin and P-selectin glycoprotein ligand-1 measured with atomic force microscopy. Colloids Surf. B Biointerfaces 2021, 207, 112002. [Google Scholar] [CrossRef]
- Zacarias-Lara, O.J.; Correa-Basurto, J.; Bello, M. Exploring the conformational and binding properties of unphosphorylated/phosphorylated monomeric and trimeric Bcl-2 through docking and molecular dynamics simulations. Biopolymers 2016, 105, 393–413. [Google Scholar] [CrossRef]
- Yang, J.; Petitjean, S.J.L.; Koehler, M.; Zhang, Q.; Dumitru, A.C.; Chen, W.; Derclaye, S.; Vincent, S.P.; Soumillion, P.; Alsteens, D. Molecular interaction and inhibition of SARS-CoV-2a binding to the ACE2 receptor. Nat. Commun. 2020, 11, 4541. [Google Scholar] [CrossRef]
- Koehler, M.; Ray, A.; Moreira, R.A.; Juniku, B.; Poma, A.B.; Alsteens, D. Molecular insights into receptor binding energetics and neutralization of SARS-CoV-2 variants. Nat. Commun. 2021, 12, 6977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hong, B.; Wei, P.; Pei, P.; Xu, H.; Chen, L.; Tong, Y.; Chen, J.; Luo, S.Z.; Fan, H.; et al. Pathogen-host adhesion between SARS-CoV-2 spike proteins from different variants and human ACE2 studied at single-molecule and single-cell levels. Emerg. Microbes Infect. 2022, 11, 2658–2669. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Huang, S.; Liu, C.; Fu, Y. Evaluating the effect of aminoglycosides on the interaction between bovine serum albumins by atomic force microscopy. Int. J. Biol. Macromol. 2019, 134, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Doherty, C.P.A.; Young, L.M.; Karamanos, T.K.; Smith, H.I.; Jackson, M.P.; Radford, S.E.; Brockwell, D.J. A peptide-display protein scaffold to facilitate single molecule force studies of aggregation-prone peptides. Protein Sci. 2018, 27, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, B.; Kamruzzahan, A.S.; Bizzarri, A.R.; Rankl, C.; Gruber, H.J.; Hinterdorfer, P.; Cannistraro, S. Single molecule recognition between cytochrome C 551 and gold-immobilized azurin by force spectroscopy. Biophys. J. 2005, 89, 2783–2791. [Google Scholar] [CrossRef]
- Perez-Dominguez, S.; Caballero-Mancebo, S.; Marcuello, C.; Martinez-Julvez, M.; Medina, M.; Lostao, A. Nanomechanical Study of Enzyme: Coenzyme Complexes: Bipartite Sites in Plastidic Ferredoxin-NADP(+) Reductase for the Interaction with NADP(+). Antioxidants 2022, 11, 537. [Google Scholar] [CrossRef]
- Alsteens, D.; Pfreundschuh, M.; Zhang, C.; Spoerri, P.M.; Coughlin, S.R.; Kobilka, B.K.; Muller, D.J. Imaging G protein-coupled receptors while quantifying their ligand-binding free-energy landscape. Nat. Methods 2015, 12, 845–851. [Google Scholar] [CrossRef]
- Friddle, R.W.; Noy, A.; De Yoreo, J.J. Interpreting the widespread nonlinear force spectra of intermolecular bonds. Proc. Natl. Acad. Sci. USA 2012, 109, 13573–13578. [Google Scholar] [CrossRef]
- Reiter-Scherer, V.; Cuellar-Camacho, J.L.; Bhatia, S.; Haag, R.; Herrmann, A.; Lauster, D.; Rabe, J.P. Force Spectroscopy Shows Dynamic Binding of Influenza Hemagglutinin and Neuraminidase to Sialic Acid. Biophys. J. 2019, 116, 1037–1048. [Google Scholar] [CrossRef]
- Lo Giudice, C.; Zhang, H.; Wu, B.; Alsteens, D. Mechanochemical Activation of Class-B G-Protein-Coupled Receptor upon Peptide-Ligand Binding. Nano Lett. 2020, 20, 5575–5582. [Google Scholar] [CrossRef] [PubMed]
- Ku, B.; Liang, C.; Jung, J.U.; Oh, B.H. Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX. Cell Res. 2011, 21, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Olivero, G.; Maiolo, D.; Leali, D.; Federici, S.; Depero, L.E.; Presta, M.; Mitola, S.; Bergese, P. Nanoliter contact angle probes tumor angiogenic ligand-receptor protein interactions. Biosens. Bioelectron. 2010, 26, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Barkai, H.; Soumya, E.; Sadiki, M.; Mounyr, B.; Ibnsouda, K.S. Impact of enzymatic treatment on wood surface free energy: Contact angle analysis. J. Adhes. Sci. Technol. 2016, 31, 726–734. [Google Scholar] [CrossRef]
- Amiri, S.; Pashizeh, F.; Moeinabadi-Bidgoli, K.; Eyvazi, Y.; Akbari, T.; Salehi Moghaddam, Z.; Eskandarisani, M.; Farahmand, F.; Hafezi, Y.; Nouri Jevinani, H.; et al. Co-encapsulation of hydrophilic and hydrophobic drugs into niosomal nanocarrier for enhanced breast cancer therapy: In silico and in vitro studies. Environ. Res. 2023, 239, 117292. [Google Scholar] [CrossRef]
- Grabowska, I.; Dehaen, W.; Radecka, H.; Radecki, J. Exploring of protein—Protein interactions at the solid—Aqueous interface by means of contact angle measurements. Colloids Surf. B Biointerfaces 2016, 141, 558–564. [Google Scholar] [CrossRef]
- Shahabadi, N.; Zendehcheshm, S. Evaluation of ct-DNA and HSA binding propensity of antibacterial drug chloroxine: Multi-spectroscopic analysis, atomic force microscopy and docking simulation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 230, 118042. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Chen, M.; Li, C.; Huang, W.; Gu, K.; Yu, H.; Yuan, Y.; Wang, Y.; Yang, B.; et al. Stepwise rapid tracking strategy to identify active molecules from Ixeris sonchifolia Hance based on “affinity mass spectrometry-atomic force microscopy imaging” technology. Talanta 2020, 217, 121031. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, Z.; Chen, P.; Zhao, Z.; Lin, C.; Zhu, C.; Wu, A. Insights on the interactions of human serum albumin with three natural phenylethanoid glycosides that inhibit HeLa cells proliferation. J. Mol. Struct. 2022, 1251, 132050. [Google Scholar] [CrossRef]
- Duan, L.; Dong, S.; Huang, K.; Cong, Y.; Luo, S.; Zhang, J.Z.H. Computational analysis of binding free energies, hotspots and the binding mechanism of Bcl-xL/Bcl-2 binding to Bad/Bax. Phys. Chem. Chem. Phys. 2021, 23, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Caro-Gomez, L.A.; Rosas-Trigueros, J.L.; Mixcoha, E.; Zamorano-Carrillo, A.; Martinez-Martinez, J.; Benitez-Cardoza, C.G. Anti-apoptotic Bcl-2 protein in apo and holo conformation anchored to the membrane: Comparative molecular dynamics simulations. J. Biomol. Struct. Dyn. 2023, 41, 6074–6088. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, J.; Sun, H.; Fu, Y.; Wang, Y. Probing Changes in Ca2+-Induced Interaction Forces between Calmodulin and Melittin by Atomic Force Microscopy. Micromachines 2020, 11, 906. [Google Scholar] [CrossRef]
- Amyot, R.; Flechsig, H. BioAFMviewer: An interactive interface for simulated AFM scanning of biomolecular structures and dynamics. PLoS Comput. Biol. 2020, 16, e1008444. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-S.; Huefner, N.D.; Chan, W.S.; Stevens, F.; Harris, J.M.; Beebe, T.P. Specific interactions between biotin and avidin studied by atomic force microscopy using the Poisson statistical analysis method. Langmuir 1999, 15, 1373–1382. [Google Scholar] [CrossRef]
- Abu-Lail, N.I.; Camesano, T.A. Specific and nonspecific interaction forces between Escherichia coli and silicon nitride, determined by Poisson statistical analysis. Langmuir 2006, 22, 7296–7301. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Van Oss, C. Acid—Base interfacial interactions in aqueous media. Colloids Surf. A Physicochem. Eng. Asp. 1993, 78, 1–49. [Google Scholar] [CrossRef]
- Van Oss, C.; Giese, R. The hydrophilicity and hydrophobicity of clay minerals. Clays Clay Miner. 1995, 43, 474–477. [Google Scholar] [CrossRef]
- van Oss, C.J.; Docoslis, A.; Giese, R.F., Jr. Free energies of protein adsorption onto mineral particles—From the initial encounter to the onset of hysteresis. Colloids Surf. B Biointerfaces 2001, 22, 285–300. [Google Scholar] [CrossRef]
- Subhi, N.; Verliefde, A.R.D.; Chen, V.; Le-Clech, P. Assessment of physicochemical interactions in hollow fibre ultrafiltration membrane by contact angle analysis. J. Membr. Sci. 2012, 403–404, 32–40. [Google Scholar] [CrossRef]






| Sample | (pN) | (pN) | koff (Feq) (S−1) | (S−1) | (nm) | (S) | −ΔGbu (kBT) |
|---|---|---|---|---|---|---|---|
| Control | 28.42 ± 3.32 | 34.25 ± 1.87 | 58.22 ± 8.30 | 25.84 ± 1.90 | 0.1178 ± 0.0036 | 0.0387 | 9.83 ± 1.87 |
| Kaempferol | 24.94 ± 2.34 | 34.49 ± 2.62 | 73.44 ± 13.56 | 36.26 ± 2.38 | 0.1191 ± 0.0088 | 0.0276 | 7.57 ± 1.42 |
| Quercetin | 22.30 ± 2.95 | 35.49 ± 4.95 | 66.66 ± 18.31 | 36.15 ± 3.65 | 0.1158 ± 0.0166 | 0.0277 | 6.05 ± 1.60 |
| Dihydromyricetin | 19.66 ± 1.46 | 35.93 ± 3.65 | 64.54 ± 12.36 | 37.95 ± 3.77 | 0.1144 ± 0.0115 | 0.0264 | 4.70 ± 0.70 |
| Baicalin | 18.74 ± 1.49 | 42.71 ± 4.19 | 87.03 ± 11.22 | 56.76 ± 3.05 | 0.0962 ± 0.0094 | 0.0176 | 4.27 ± 0.68 |
| Curcumin | 18.05 ± 1.66 | 38.24 ± 4.32 | 70.53 ± 11.51 | 44.62 ± 3.14 | 0.1075 ± 0.0124 | 0.0224 | 3.96 ± 0.73 |
| Rutin | 15.27 ± 2.47 | 43.81 ± 16.25 | 106.64 ± 36.90 | 76.07 ± 13.87 | 0.09381 ± 0.0313 | 0.0131 | 2.84 ± 0.92 |
| EGCG | 16.73 ± 0.74 | 40.18 ± 4.98 | 105.19 ± 17.33 | 70.25 ± 6.93 | 0.1023 ± 0.0125 | 0.0142 | 3.41 ± 0.30 |
| Gossypol | 22.19 ± 3.89 | 40.56 ± 4.53 | 79.63 ± 20.15 | 46.66 ± 4.70 | 0.1013 ± 0.0112 | 0.0214 | 5.99 ± 2.10 |
| Sample | τ (ms) | (M−1s−1) | (M) |
|---|---|---|---|
| Control | 72.89 ± 10.20 | 7.96 ± 0.93 × 105 | 3.25 ± 0.44 × 10−5 |
| Kaempferol | 113.36 ± 21.82 | 5.12 ± 0.84 × 105 | 7.09 ± 1.25 × 10−5 |
| Quercetin | 122.57 ± 39.35 | 4.73 ± 1.41 × 105 | 7.64 ± 2.88 × 10−5 |
| Dihydromyricetin | 116.37 ± 37.45 | 4.98 ± 1.49 × 105 | 7.61 ± 2.39 × 10−5 |
| Baicalin | 478.29 ± 78.60 | 1.21 ± 0.17 × 105 | 4.68 ± 0.70 × 10−4 |
| Curcumin | 173.20 ± 30.61 | 3.35 ± 0.50 × 105 | 1.33 ± 0.20 × 10−4 |
| Rutin | 317.91 ± 29.84 | 1.82 ± 0.14 × 105 | 4.17 ± 0.83 × 10−4 |
| EGCG | 259.54 ± 92.36 | 2.23 ± 0.75 × 105 | 3.14 ± 0.74 × 10−4 |
| Gossypol | 127.16 ± 32.89 | 4.56 ± 1.04 × 105 | 1.02 ± 0.38 × 10−4 |
| Polyphenols | The Number of Interacting Amino Acids | Amino Acids Forming H-Bond | Amino Acids Involved in van der Waals Interactions | Amino Acids Involved in Hydrophobic Interactions | Binding Energy (kcal/mol) |
|---|---|---|---|---|---|
| Kaempferol | 11 | Leu137 | Ala149, Tyr108, Phe153, Val156, Glu152, Phe112, Ser105, Phe104, Glu136 | Met115, Leu137 | −26.47 |
| Quercetin | 11 | Phe112, Val133, Leu137, Glu152 | Leu137, Glu136, Asp111, Val133, Phe112 | Phe104, Met115, Val133, Leu137, Ala149, Phe153, Val156 | −27.72 |
| Dihydromyricetin | 12 | Glu152, Phe153 | Gln118, Ser116, Glu114, Phe112, Phe104, Asp111, Leu137, Tyr108 | Met115, Val156 | −27.50 |
| Baicalin | 13 | Tyr108, Gly145, Arg146 | Leu137, Ala149, Asn143, Phe104, Glu152, Ser105, Phe112, Phe153 | Tyr108, Met115, Val156 | −41.32 |
| Curcumin | 16 | Tyr108, Trp144, Ala149 | Ala100, Arg107, Phe198, Gly145, Arg146, Asp111, Leu137, Phe150, Val133, Phe153, Met115 | Phe104, Val148, Ala149 | −33.66 |
| Rutin | 21 | Asp111, Leu137, Arg139, Arg146, Ala149 | Glu152, Phe150, Phe153, Val156, Phe112, Met115, Tyr108, Val133, Gly145, Asn143, Asp140, Phe138, Gly141, Glu136 | Phe104, Leu137, Arg146, Ala149 | −64.85 |
| EGCG | 17 | Tyr108, Leu137, Asp140, | Glu152, Val156, Phe112, Phe153, Val133, Asp111, Phe104, Glu136, ARG139, Gly141, Phe138, Arr146 | Tyr108, Met115, Leu137, Ala149 | −56.17 |
| Gossypol | 14 | Met115, Glu136, Leu137 | Glu114, Val153, Phe112, Glu152, Ala149, Arg146, Tyr108, Phe138, Asp 140 | Phe104, Asp111, Met115, Leu137, Phe153 | −34.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Liao, F.; Tian, Y.; Lei, Y.; Fu, Y.; Wang, J. Molecular-Scale Investigations Reveal the Effect of Natural Polyphenols on BAX/Bcl-2 Interactions. Int. J. Mol. Sci. 2024, 25, 2474. https://doi.org/10.3390/ijms25052474
Sun H, Liao F, Tian Y, Lei Y, Fu Y, Wang J. Molecular-Scale Investigations Reveal the Effect of Natural Polyphenols on BAX/Bcl-2 Interactions. International Journal of Molecular Sciences. 2024; 25(5):2474. https://doi.org/10.3390/ijms25052474
Chicago/Turabian StyleSun, Heng, Fenghui Liao, Yichen Tian, Yongrong Lei, Yuna Fu, and Jianhua Wang. 2024. "Molecular-Scale Investigations Reveal the Effect of Natural Polyphenols on BAX/Bcl-2 Interactions" International Journal of Molecular Sciences 25, no. 5: 2474. https://doi.org/10.3390/ijms25052474