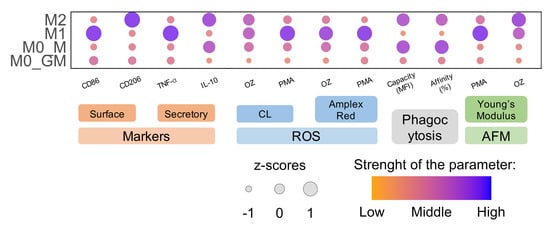
Radical-Generating Activity, Phagocytosis, and Mechanical Properties of Four Phenotypes of Human Macrophages
Abstract
:1. Introduction
2. Results
2.1. Monocyte Differentiation and Macrophage Polarization
2.2. Effect of Growth Factors and Polarization Inducers on Macrophage Survival
2.3. ROS Generation by Activated Macrophages
2.3.1. Luminol-Dependent Chemiluminescence
2.3.2. Oxidation of Amplex Red by Horseradish Peroxidase in the Presence of Activated Macrophages
2.4. Comparison of ROS-Generating Ability of M1 and M2 Macrophages
2.5. Macrophage Phagocytosis
2.6. Changes in Mechanical Properties of Macrophages after Activation
3. Discussion
4. Materials and Methods
4.1. Cell Culturing
4.2. Measurement of Surface Markers of Macrophage Polarization
4.3. Measurement of Secreted Markers of Macrophage Polarization
4.4. PicoGreen Assay
4.5. Measurements of ROS Production by Macrophages
4.6. Phagocytosis Analysis
4.7. Atomic Force Microscopy
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 2008, 8, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Ushach, I.; Zlotnik, A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J. Leukoc. Biol. 2016, 100, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.; Mantovani, A.; Sica, A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv. Immunol. 2013, 120, 163–184. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Orekhova, V.A.; Nikiforov, N.G.; Myasoedova, V.A.; Grechko, A.V.; Romanenko, E.B.; Dongwei, Z.; Chistiakov, D.A. Monocyte differentiation and macrophage polarization. Vessel Plus 2019, 2019, 10. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Mills, C.D.; Ley, K. M1 and M2 macrophages: The chicken and the egg of immunity. J. Innate Immun. 2014, 6, 716–726. [Google Scholar] [CrossRef]
- Hamidzadeh, K.; Belew, A.T.; El-Sayed, N.M.; Mosser, D.M. The transition of M-CSF-derived human macrophages to a growth-promoting phenotype. Blood Adv. 2020, 4, 5460–5472. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Rius-Pérez, S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants 2022, 11, 1394. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Mushenkova, N.V.; Nikiforov, N.G.; Shakhpazyan, N.K.; Orekhova, V.A.; Sadykhov, N.K.; Orekhov, A.N. Phenotype Diversity of Macrophages in Osteoarthritis: Implications for Development of Macrophage Modulating Therapies. Int. J. Mol. Sci. 2022, 23, 8381. [Google Scholar] [CrossRef] [PubMed]
- Kadomoto, S.; Izumi, K.; Mizokami, A. Macrophage Polarity and Disease Control. Int. J. Mol. Sci. 2021, 23, 144. [Google Scholar] [CrossRef] [PubMed]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef]
- Zhang, Y.; Choksi, S.; Chen, K.; Pobezinskaya, Y.; Linnoila, I.; Liu, Z.G. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013, 23, 898–914. [Google Scholar] [CrossRef]
- Griess, B.; Mir, S.; Datta, K.; Teoh-Fitzgerald, M. Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free Radic. Biol. Med. 2020, 147, 48–60. [Google Scholar] [CrossRef]
- Nauseef, W.M. The phagocyte NOX2 NADPH oxidase in microbial killing and cell signaling. Curr. Opin. Immunol. 2019, 60, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. The biological chemistry of hydrogen peroxide. Methods Enzymol. 2013, 528, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, J. Host-Derived Cytotoxic Agents in Chronic Inflammation and Disease Progression. Int. J. Mol. Sci. 2023, 24, 3016. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.S.; O’Brien-Simpson, N.M.; Holden, J.A.; Lenzo, J.C.; Fong, S.B.; Reynolds, E.C. Unprimed, M1 and M2 Macrophages Differentially Interact with Porphyromonas gingivalis. PLoS ONE 2016, 11, e0158629. [Google Scholar] [CrossRef]
- Balce, D.R.; Li, B.; Allan, E.R.; Rybicka, J.M.; Krohn, R.M.; Yates, R.M. Alternative activation of macrophages by IL-4 enhances the proteolytic capacity of their phagosomes through synergistic mechanisms. Blood 2011, 118, 4199–4208. [Google Scholar] [CrossRef]
- Varin, A.; Mukhopadhyay, S.; Herbein, G.; Gordon, S. Alternative activation of macrophages by IL-4 impairs phagocytosis of pathogens but potentiates microbial-induced signalling and cytokine secretion. Blood 2010, 115, 353–362. [Google Scholar] [CrossRef]
- Schulz, D.; Severin, Y.; Zanotelli, V.R.T.; Bodenmiller, B. In-Depth Characterization of Monocyte-Derived Macrophages using a Mass Cytometry-Based Phagocytosis Assay. Sci. Rep. 2019, 9, 1925. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, Z.; Sahni, S.; Lok, H.; Davies, M.J.; Wink, D.A.; Richardson, D.R. Regulation and control of nitric oxide (NO) in macrophages: Protecting the “professional killer cell” from its own cytotoxic arsenal via MRP1 and GSTP1. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 995–999. [Google Scholar] [CrossRef]
- Schneemann, M.; Schoedon, G.; Hofer, S.; Blau, N.; Guerrero, L.; Schaffner, A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J. Infect. Dis. 1993, 167, 1358–1363. [Google Scholar] [CrossRef]
- Schneemann, M.; Schoeden, G. Macrophage biology and immunology: Man is not a mouse. J. Leukoc. Biol. 2007, 81, 579. [Google Scholar] [CrossRef]
- Weinberg, J.B.; Misukonis, M.A.; Shami, P.J.; Mason, S.N.; Sauls, D.L.; Dittman, W.A.; Wood, E.R.; Smith, G.K.; McDonald, B.; Bachus, K.E. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): Analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood 1995, 86, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Neves-Souza, P.C.; Azeredo, E.L.; Zagne, S.M.; Valls-de-Souza, R.; Reis, S.R.; Cerqueira, D.I.; Nogueira, R.M.; Kubelka, C.F. Inducible nitric oxide synthase (iNOS) expression in monocytes during acute Dengue Fever in patients and during in vitro infection. BMC Infect. Dis. 2005, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.V.; Vinh, A.; Diep, H.; Samuel, C.S.; Drummond, G.R.; Kemp-Harper, B.K. Distinct Redox Signalling following Macrophage Activation Influences Profibrotic Activity. J. Immunol. Res. 2019, 2019, 1278301. [Google Scholar] [CrossRef]
- Nassif, R.M.; Chalhoub, E.; Chedid, P.; Hurtado-Nedelec, M.; Raya, E.; Dang, P.M.C.; Jean-Claude, M.; El-Benna, J. Metformin Inhibits ROS Production by Human M2 Macrophages via the Activation of AMPK. Biomedicines 2022, 10, 319. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Yuan, Y.; Huang, B. Applications of atomic force microscopy in immunology. Front. Med. 2021, 15, 43–52. [Google Scholar] [CrossRef]
- Pi, J.; Li, T.; Liu, J.; Su, X.; Wang, R.; Yang, F.; Bai, H.; Jin, H.; Cai, J. Detection of lipopolysaccharide induced inflammatory responses in RAW264.7 macrophages using atomic force microscope. Micron 2014, 65, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leporatti, S.; Gerth, A.; Köhler, G.; Kohlstrunk, B.; Hauschildt, S.; Donath, E. Elasticity and adhesion of resting and lipopolysaccharide-stimulated macrophages. FEBS Lett. 2006, 580, 450–454. [Google Scholar] [CrossRef]
- Patel, N.R.; Bole, M.; Chen, C.; Hardin, C.C.; Kho, A.T.; Mih, J.; Deng, L.; Butler, J.; Tschumperlin, D.; Fredberg, J.J.; et al. Cell elasticity determines macrophage function. PLoS ONE 2012, 7, e41024. [Google Scholar] [CrossRef]
- Thornton, S.; Tan, R.; Sproles, A.; Do, T.; Schick, J.; Grom, A.A.; DeLay, M.; Schulert, G.S. A Multiparameter Flow Cytometry Analysis Panel to Assess CD163 mRNA and Protein in Monocyte and Macrophage Populations in Hyperinflammatory Diseases. J. Immunol. 2019, 202, 1635–1643. [Google Scholar] [CrossRef]
- Hu, L.; Zachariae, E.D.; Larsen, U.G.; Vilhardt, F.; Petersen, S.V. The dynamic uptake and release of SOD3 from intracellular stores in macrophages modulates the inflammatory response. Redox. Biol. 2019, 26, 101268. [Google Scholar] [CrossRef]
- Jancinová, V.; Drábiková, K.; Nosál, R.; Racková, L.; Májeková, M.; Holománová, D. The combined luminol/isoluminol chemiluminescence method for differentiating between extracellular and intracellular oxidant production by neutrophils. Redox. Rep. 2006, 11, 110–116. [Google Scholar] [CrossRef]
- Hoppenbrouwers, T.; Bastiaan-Net, S.; Garssen, J.; Pellegrini, N.; Willemsen, L.E.; Wichers, H.J. Functional differences between primary monocyte-derived and THP-1 macrophages and their response to LCPUFAs. PharmaNutrition 2022, 22, 100322. [Google Scholar] [CrossRef]
- Tedesco, S.; De Majo, F.; Kim, J.; Trenti, A.; Trevisi, L.; Fadini, G.P.; Bolego, C.; Zandstra, P.W.; Cignarella, A.; Vitiello, L. Convenience versus Biological Significance: Are PMA-Differentiated THP-1 Cells a Reliable Substitute for Blood-Derived Macrophages When Studying. Front. Pharmacol. 2018, 9, 71. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Velay-Lizancos, M.; Weaver, C.J.; Athamneh, A.I.; Zavattieri, P.D.; Suter, D.M.; Raman, A. Anisotropy vs isotropy in living cell indentation with AFM. Sci. Rep. 2019, 9, 5757. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Wrona, E.A.; Romero-Torres, S.; Pallotta, I.; Graney, P.L.; Witherel, C.E.; Panicker, L.M.; Feldman, R.A.; Urbanska, A.M.; Santambrogio, L.; et al. Differential gene expression in human, murine, and cell line-derived macrophages upon polarization. Exp. Cell Res. 2016, 347, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hao, Z.; Wu, J.; Ma, C.; Xu, Y.; Li, J.; Lan, R.; Zhu, B.; Ren, P.; Fan, D.; et al. Comparative Proteomic Analysis of Polarized Human THP-1 and Mouse RAW264.7 Macrophages. Front. Immunol. 2021, 12, 700009. [Google Scholar] [CrossRef] [PubMed]
- Qie, J.; Liu, Y.; Wang, Y.; Zhang, F.; Qin, Z.; Tian, S.; Liu, M.; Li, K.; Shi, W.; Song, L.; et al. Integrated proteomic and transcriptomic landscape of macrophages in mouse tissues. Nat. Commun. 2022, 13, 7389. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Gallerand, A.; Erlich, E.C.; Helmink, B.A.; Mair, I.; Li, X.; Eckhouse, S.R.; Dimou, F.M.; Shakhsheer, B.A.; Phelps, H.M.; et al. Human serous cavity macrophages and dendritic cells possess counterparts in the mouse with a distinct distribution between species. Nat. Immunol. 2024, 25, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Zajd, C.M.; Ziemba, A.M.; Miralles, G.M.; Nguyen, T.; Feustel, P.J.; Dunn, S.M.; Gilbert, R.J.; Lennartz, M.R. Bone Marrow-Derived and Elicited Peritoneal Macrophages Are Not Created Equal: The Questions Asked Dictate the Cell Type Used. Front. Immunol. 2020, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Taylor, L.; Lee, H.; Cowley, S.A.; James, W.S.; Iqbal, A.J.; Greaves, D.R. A novel real time imaging platform to quantify macrophage phagocytosis. Biochem. Pharmacol. 2016, 116, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Härtlova, A.; Dill, B.D.; Prescott, A.R.; Gierliński, M.; Trost, M. High-resolution quantitative proteome analysis reveals substantial differences between phagosomes of RAW 264.7 and bone marrow derived macrophages. Proteomics 2015, 15, 3169–3174. [Google Scholar] [CrossRef]
- Huang, N.N.; Becker, S.; Boularan, C.; Kamenyeva, O.; Vural, A.; Hwang, I.Y.; Shi, C.S.; Kehrl, J.H. Canonical and noncanonical g-protein signaling helps coordinate actin dynamics to promote macrophage phagocytosis of zymosan. Mol. Cell. Biol. 2014, 34, 4186–4199. [Google Scholar] [CrossRef]
- Krombach, F.; Münzing, S.; Allmeling, A.M.; Gerlach, J.T.; Behr, J.; Dörger, M. Cell size of alveolar macrophages: An interspecies comparison. Environ. Health Perspect. 1997, 105, 1261–1263. [Google Scholar] [CrossRef]
- Vijayan, V.; Pradhan, P.; Braud, L.; Fuchs, H.R.; Gueler, F.; Motterlini, R.; Foresti, R.; Immenschuh, S. Human and murine macrophages exhibit differential metabolic responses to lipopolysaccharide—A divergent role for glycolysis. Redox. Biol. 2019, 22, 101147. [Google Scholar] [CrossRef]
- Sieweke, M.H.; Allen, J.E. Beyond stem cells: Self-renewal of differentiated macrophages. Science 2013, 342, 1242974. [Google Scholar] [CrossRef]
- Lacey, D.C.; Achuthan, A.; Fleetwood, A.J.; Dinh, H.; Roiniotis, J.; Scholz, G.M.; Chang, M.W.; Beckman, S.K.; Cook, A.D.; Hamilton, J.A. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J. Immunol. 2012, 188, 5752–5765. [Google Scholar] [CrossRef]
- Shalhoub, V.; Faust, J.; Boyle, W.J.; Dunstan, C.R.; Kelley, M.; Kaufman, S.; Scully, S.; Van, G.; Lacey, D.L. Osteoprotegerin and osteoprotegerin ligand effects on osteoclast formation from human peripheral blood mononuclear cell precursors. J. Cell. Biochem. 1999, 72, 251–261. [Google Scholar] [CrossRef]
- Unuvar Purcu, D.; Korkmaz, A.; Gunalp, S.; Helvaci, D.G.; Erdal, Y.; Dogan, Y.; Suner, A.; Wingender, G.; Sag, D. Effect of stimulation time on the expression of human macrophage polarization markers. PLoS ONE 2022, 17, e0265196. [Google Scholar] [CrossRef] [PubMed]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.; Dockrell, D.H. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef] [PubMed]
- Ledo, A.; Fernandes, E.; Salvador, A.; Laranjinha, J.; Barbosa, R.M. In vivo hydrogen peroxide diffusivity in brain tissue supports volume signaling activity. Redox. Biol. 2022, 50, 102250. [Google Scholar] [CrossRef] [PubMed]
- Müllebner, A.; Dorighello, G.G.; Kozlov, A.V.; Duvigneau, J.C. Interaction between Mitochondrial Reactive Oxygen Species, Heme Oxygenase, and Nitric Oxide Synthase Stimulates Phagocytosis in Macrophages. Front. Med. 2017, 4, 252. [Google Scholar] [CrossRef]
- Gantner, B.N.; Simmons, R.M.; Canavera, S.J.; Akira, S.; Underhill, D.M. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 2003, 197, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A. GM-CSF in inflammation. J. Exp. Med. 2020, 217, e20190945. [Google Scholar] [CrossRef] [PubMed]
- Chernykh, E.R.; Sakhno, L.V.; Shevela, E.Y.; Tikhonova, M.A.; Khonina, N.A.; Ostanin, A.A. Phenotypic and functional changes of GM-CSF differentiated human macrophages following exposure to apoptotic neutrophils. Cell. Immunol. 2018, 331, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.; Kzhyshkowska, J.; Nurgazieva, D.; Goerdt, S.; Gratchev, A. Pro- and anti-inflammatory control of M-CSF-mediated macrophage differentiation. Immunobiology 2011, 216, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Kraaij, M.D.; Koekkoek, K.M.; van der Kooij, S.W.; Gelderman, K.A.; van Kooten, C. Subsets of human type 2 macrophages show differential capacity to produce reactive oxygen species. Cell. Immunol. 2013, 284, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jaguin, M.; Houlbert, N.; Fardel, O.; Lecureur, V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell. Immunol. 2013, 281, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.E.; Lewis, C.V.; Zhu, M.; Wang, C.; Samuel, C.S.; Drummond, G.R.; Kemp-Harper, B.K. IL-4 and IL-13 induce equivalent expression of traditional M2 markers and modulation of reactive oxygen species in human macrophages. Sci. Rep. 2023, 13, 19589. [Google Scholar] [CrossRef]
- Souza, S.T.; Agra, L.C.; Santos, C.E.; Barreto, E.; Hickmann, J.M.; Fonseca, E.J. Macrophage adhesion on fibronectin evokes an increase in the elastic property of the cell membrane and cytoskeleton: An atomic force microscopy study. Eur. Biophys. J. 2014, 43, 573–579. [Google Scholar] [CrossRef]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015, 15, 1–14. [Google Scholar] [CrossRef]
- Murashko, A.V.; Frolova, A.A.; Akovantseva, A.A.; Kotova, S.L.; Timashev, P.S.; Efremov, Y.M. The cell softening as a universal indicator of cell damage during cytotoxic effects. Biochim. Biophys. Acta Gen. Subj. 2023, 1867, 130348. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhang, B.; Tang, G.; Yu, X.F.; Galluzzi, M. Cells nanomechanics by atomic force microscopy: Focus on interactions at nanoscale. Adv. Phys. X 2021, 6, 1866668. [Google Scholar] [CrossRef]
- Venkatachalam, G.; Arumugam, S.; Doble, M. Synthesis, Characterization, and Biological Activity of Aminated Zymosan. ACS Omega 2020, 5, 15973–15982. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, I.I.; Suleimanov, S.K.; Mikhalchik, E.V.; Urmantaeva, N.T.; Salimov, E.L.; Ragimov, A.A.; Khlebnikova, T.M.; Timashev, P.S. Redox-Activation of Neutrophils Induced by Pericardium Scaffolds. Int. J. Mol. Sci. 2022, 23, 15468. [Google Scholar] [CrossRef]
- Schillers, H.; Rianna, C.; Schäpe, J.; Luque, T.; Doschke, H.; Wälte, M.; Uriarte, J.J.; Campillo, N.; Michanetzis, G.P.; Bobrowska, J. Standardized nanomechanical atomic force microscopy procedure (SNAP) for measuring soft and biological samples. Scientific reports 2017, 1, 5117. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Wang, W.H.; Hardy, S.D.; Geahlen, R.L.; Raman, A. Measuring nanoscale viscoelastic parameters of cells directly from AFM force-displacement curves. Sci. Rep. 2017, 7, 1541. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Shpichka, A.I.; Kotova, S.L.; Timashev, P.S. Viscoelastic mapping of cells based on fast force volume and PeakForce Tapping. Soft Matter 2019, 15, 5455–5463. [Google Scholar] [CrossRef] [PubMed]
- Ting, T.C.T. The contact stresses between a rigid indenter and a viscoelastic half-space. J. Appl. Mech. 1966, 4, 845–854. [Google Scholar] [CrossRef]
- Kollmannsberger, P.; Fabry, B. Active soft glassy rheology of adherent cells. Soft Matter 2009, 9, 1771–1774. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Okajima, T.; Raman, A. Measuring viscoelasticity of soft biological samples using atomic force microscopy. Soft Matter 2020, 5, 64–81. [Google Scholar] [CrossRef]
- Smith, T.D.; Tse, M.J.; Read, E.L.; Liu, W.F. Regulation of macrophage polarization and plasticity by complex activation signals. Integr. Biol. 2016, 16, 946–955. [Google Scholar] [CrossRef] [PubMed]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suleimanov, S.K.; Efremov, Y.M.; Klyucherev, T.O.; Salimov, E.L.; Ragimov, A.A.; Timashev, P.S.; Vlasova, I.I. Radical-Generating Activity, Phagocytosis, and Mechanical Properties of Four Phenotypes of Human Macrophages. Int. J. Mol. Sci. 2024, 25, 1860. https://doi.org/10.3390/ijms25031860
Suleimanov SK, Efremov YM, Klyucherev TO, Salimov EL, Ragimov AA, Timashev PS, Vlasova II. Radical-Generating Activity, Phagocytosis, and Mechanical Properties of Four Phenotypes of Human Macrophages. International Journal of Molecular Sciences. 2024; 25(3):1860. https://doi.org/10.3390/ijms25031860
Chicago/Turabian StyleSuleimanov, Shakir K., Yuri M. Efremov, Timofey O. Klyucherev, Emin L. Salimov, Aligeydar A. Ragimov, Peter S. Timashev, and Irina I. Vlasova. 2024. "Radical-Generating Activity, Phagocytosis, and Mechanical Properties of Four Phenotypes of Human Macrophages" International Journal of Molecular Sciences 25, no. 3: 1860. https://doi.org/10.3390/ijms25031860