The Anti-Inflammatory Potential of an Ethanolic Extract from Sarcopoterium spinosum Fruits for Protection and/or Counteraction against Oxidative Stress in Dysfunctional Endothelial Cells
Abstract
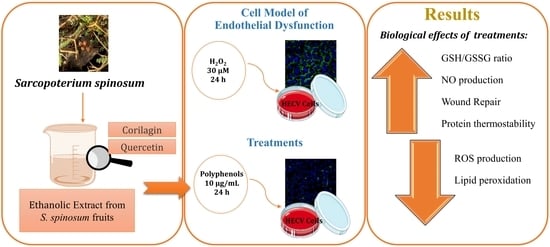
:1. Introduction
2. Results
2.1. Cytoprotective Activity of SEE on Endothelial Cells
2.2. Antioxidant Activity of SEE on Dysfunctional Endothelial Cells
2.3. Anti-Inflammatory Activity of SEE on Dysfunctional Endothelial Cells
2.4. Promoting Effect of SEE on the Wound Repair of Endothelial Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Collection and Extraction
4.3. Protein Quantification
4.4. Cell Culture and Treatments
- Counteraction Condition: The cells were first subjected to stimulation with H2O2 (30 µM for 24 h) to induce oxidative stress. Subsequently, they underwent post-treatment with either SEE, Cg, or Qu (10 µg/mL for 24 h);
- Protection Condition: The cells were pre-treated with either SEE, Cg, or Qu (10 µg/mL for 24 h) before being exposed to H2O2 (30 µM for 24 h);
- Non-treated normal cells (Ctrl) served as a crucial control to validate the efficacy of our experimental model. Furthermore, H2O2-insulted untreated cells (H2O2) were included as an inner control to compare and validate the outcomes. Each experiment was performed at least in quadruplicate.
4.5. Cell Viability Assessment
4.6. ROS Production
4.7. Lipid Peroxidation
4.8. Nitrite/Nitrate Levels
4.9. GSH/GSSG Ratio
4.10. Wound Healing Assay
4.11. Protein Denaturation Assay
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clapp, B.R.; Hingorani, A.D.; Kharbanda, R.K.; Mohamed-Ali, V.; Stephens, J.W.; Vallance, P.; MacAllister, R.J. Inflammation-Induced Endothelial Dysfunction Involves Reduced Nitric Oxide Bioavailability and Increased Oxidant Stress. Cardiovasc. Res. 2004, 64, 172–178. [Google Scholar] [CrossRef]
- Mahjoub, S.; Roudsari, J.M. Role of Oxidative Stress in Pathogenesis of Metabolic Syndrome. Casp. J. Intern. Med. 2012, 3, 386. [Google Scholar]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Rons, J.; Karina Aranda-Rivera, A.; Cruz-Gregorio, A.; Lyzet Arancibia-Hernández, Y.; Yaquelin Hernández-Cruz, E.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Mendez-Encinas, M.A.; Valencia, D.; Ortega-García, J.; Carvajal-Millan, E.; Díaz-Ríos, J.C.; Mendez-Pfeiffer, P.; Soto-Bracamontes, C.M.; Garibay-Escobar, A.; Alday, E.; Velazquez, C. Anti-Inflammatory Potential of Seasonal Sonoran Propolis Extracts and Some of Their Main Constituents. Molecules 2023, 28, 4496. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, D.; Bronas, U. Reactive Oxygen and Nitrogen Species: Impact on Endothelial Dysfunction. Nurs. Res. 2015, 64, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative Stress and Reactive Oxygen Species in Endothelial Dysfunction Associated with Cardiovascular and Metabolic Diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Domingueti, C.P.; Dusse, L.M.S.A.; Carvalho, M.D.G.; De Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes Mellitus: The Linkage between Oxidative Stress, Inflammation, Hypercoagulability and Vascular Complications. J. Diabetes Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Di Mauro, M.D.; Fava, G.; Spampinato, M.; Aleo, D.; Melilli, B.; Saita, M.G.; Centonze, G.; Maggiore, R.; D’antona, N. Polyphenolic Fraction from Olive Mill Wastewater: Scale-Up and in Vitro Studies for Ophthalmic Nutraceutical Applications. Antioxidants 2019, 8, 462. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and Disease Risk in Epidemiologic Studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, Flavones, Flavanones, and Human Health: Epidemiological Evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Al-Aboudi, A.; Afifi, F.U. Plants Used for the Treatment of Diabetes in Jordan: A Review of Scientific Evidence. Pharm. Biol. 2011, 49, 221–239. [Google Scholar] [CrossRef]
- Charles, I.; Chaar, A. Medicinal Plants of Lebanon. Archaeol. Hist. Lebanon 2004, 19, 70–85. [Google Scholar]
- Dafni, A.; Yaniv, Z.; Palevitch, D. Ethnobotanical Survey of Medicinal Plants in Northern Israel. J. Ethnopharmacol. 1984, 10, 295–310. [Google Scholar] [CrossRef]
- Friedman, J.; Yaniv, Z.; Dafni, A.; Palewitch, D. A Preliminary Classification of the Healing Potential of Medicinal Plants, Based on a Rational Analysis of an Ethnopharmacological Field Survey among Bedouins in the Negev Desert, Israel. J. Ethnopharmacol. 1986, 16, 275–287. [Google Scholar] [CrossRef]
- Smirin, P.; Taler, D.; Abitbol, G.; Brutman-Barazani, T.; Kerem, Z.; Sampson, S.R.; Rosenzweig, T. Sarcopoterium Spinosum Extract as an Antidiabetic Agent: In Vitro and in Vivo Study. J. Ethnopharmacol. 2010, 129, 10–17. [Google Scholar] [CrossRef]
- Rozenberg, K.; Rosenzweig, T. Sarcopoterium Spinosum Extract Improved Insulin Sensitivity in Mice Models of Glucose Intolerance and Diabetes. PLoS ONE 2018, 13, e0196736. [Google Scholar] [CrossRef] [PubMed]
- Zbeeb, H.; Khalifeh, H.; Lupidi, G.; Baldini, F.; Zeaiter, L.; Khalil, M.; Salis, A.; Damonte, G.; Vergani, L. Polyphenol-Enriched Extracts of Sarcopoterium Spinosum Fruits for Counteracting Lipid Accumulation and Oxidative Stress in an in Vitro Model of Hepatic Steatosis. Fitoterapia 2023, 172, 105743. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Konukoglu, D.; Uzun, H. Endothelial Dysfunction and Hypertension. Adv. Exp. Med. Biol. 2017, 956, 511–540. [Google Scholar] [CrossRef] [PubMed]
- Vergani, L.; Vecchione, G.; Baldini, F.; Grasselli, E.; Voci, A.; Portincasa, P.; Ferrari, P.F.; Aliakbarian, B.; Casazza, A.A.; Perego, P. Polyphenolic Extract Attenuates Fatty Acid-Induced Steatosis and Oxidative Stress in Hepatic and Endothelial Cells. Eur. J. Nutr. 2018, 57, 1793–1805. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Khalifeh, H.; Saad, F.; Serale, N.; Salis, A.; Damonte, G.; Lupidi, G.; Daher, A.; Vergani, L. Protective Effects of Extracts from Ephedra Foeminea Forssk Fruits against Oxidative Injury in Human Endothelial Cells. J. Ethnopharmacol. 2020, 260, 112976. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Aboul-Enein, B.H.; Duchnik, E.; Marchlewicz, M. Antioxidative Properties of Phenolic Compounds and Their Effect on Oxidative Stress Induced by Severe Physical Exercise. J. Physiol. Sci. 2022, 72, 19. [Google Scholar] [CrossRef]
- Averill-Bates, D.A. The Antioxidant Glutathione. Vitam. Horm. 2023, 121, 109–141. [Google Scholar] [CrossRef]
- Gaucher, C.; Boudier, A.; Bonetti, J.; Clarot, I.; Leroy, P.; Parent, M. Glutathione: Antioxidant Properties Dedicated to Nanotechnologies. Antioxidants 2018, 7, 62. [Google Scholar] [CrossRef]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox Status Expressed as GSH:GSSG Ratio as a Marker for Oxidative Stress in Paediatric Tumour Patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef]
- Billack, B. Macrophage Activation: Role of Toll-like Receptors, Nitric Oxide, and Nuclear Factor Kappa B. Am. J. Pharm. Educ. 2006, 70, 102. [Google Scholar] [CrossRef]
- Förstermann, U. Oxidative Stress in Vascular Disease: Causes, Defense Mechanisms and Potential Therapies. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 338–349. [Google Scholar] [CrossRef]
- Wallace, J.L. Nitric Oxide as a Regulator of Inflammatory Processes. Mem. Inst. Oswaldo Cruz 2005, 100 (Suppl. S1), 5–9. [Google Scholar] [CrossRef] [PubMed]
- Grande, S.; Bogani, P.; De Saizieu, A.; Schueler, G.; Galli, C.; Visioli, F. Vasomodulating Potential of Mediterranean Wild Plant Extracts. J. Agric. Food Chem. 2004, 52, 5021–5026. [Google Scholar] [CrossRef] [PubMed]
- Dharmadeva, S.; Galgamuwa, L.S.; Prasadinie, C.; Kumarasinghe, N. In Vitro Anti-Inflammatory Activity of Ficus racemosa L. Bark Using Albumin Denaturation Method. Ayu 2018, 39, 239. [Google Scholar] [CrossRef]
- Horwitz, R.; Webb, D. Cell Migration. Curr. Biol. 2003, 13, R756–R759. [Google Scholar] [CrossRef]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell Migration: Integrating Signals from Front to Back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef]
- Velnar, T.; Gradisnik, L. Tissue Augmentation in Wound Healing: The Role of Endothelial and Epithelial Cells. Med. Arch. (Sarajevo Bosnia Herzeg.) 2018, 72, 444–448. [Google Scholar] [CrossRef]
- Tonin, T.D.; Thiesen, L.C.; de Oliveira Nunes, M.L.; Broering, M.F.; Donato, M.P.; Goss, M.J.; Petreanu, M.; Niero, R.; Machado, I.D.; Santin, J.R. Rubus Imperialis (Rosaceae) Extract and Pure Compound Niga-Ichigoside F1: Wound Healing and Anti-Inflammatory Effects. Naunyn. Schmiedebergs Arch. Pharmacol. 2016, 389, 1235–1244. [Google Scholar] [CrossRef]
- Hanbisa, S.; Tadesse, W.T.; Abula, T. Evaluation of Wound Healing Activity of 80% Methanol Stem-Bark Extract and Solvent Fractions of Prunus Africana (Hook.f.) Kalkman (Rosaceae) in Mice. J. Exp. Pharmacol. 2023, 15, 349–365. [Google Scholar] [CrossRef]
- Melguizo-rodríguez, L.; de Luna-Bertos, E.; Ramos-torrecillas, J.; Illescas-montesa, R.; Costela-ruiz, V.J.; García-martínez, O. Potential Effects of Phenolic Compounds That Can Be Found in Olive Oil on Wound Healing. Foods 2021, 10, 1642. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring Reactive Species and Oxidative Damage in Vivo and in Cell Culture: How Should You Do It and What Do the Results Mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef]
- Iguchi, H.; Kojo, S.; Ikeda, M. Lipid Peroxidation and Disintegration of the Cell Membrane Structure in Cultures of Rat Lung Fibroblasts Treated with Asbestos. J. Appl. Toxicol. 1993, 13, 269–275. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of Nitrate, Nitrite, and [15N]Nitrate in Biological Fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Rodriguez, L.G.; Wu, X.; Guan, J.L. Wound-Healing Assay. Methods Mol. Biol. 2005, 294, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.A.D.; Roesner, H.; Conrad, J.; Moeller, W.; Beifuss, U.; Chiba, K.; Nkurunziza, J.P.; Kraus, W. Selected Secondary Metabolites from the Phytolaccaceae and Their Biological/Pharmaceutical Significance. Phytochemistry 2002, 6, 13–68. [Google Scholar]





| RT (min) | [M-H]− | MS/MS Fragments | Identified PPs | Classification | % |
|---|---|---|---|---|---|
| 13.5 | 935 | 633/301/897 | Casuarictin isomer | Ellagitannins | 15.0 |
| 15.4 | 935 | 633/301/897/783 | Casuarictin isomer | Ellagitannins | 8.9 |
| 17.9 | 477.1 | 301 | Quercetin glucuronide | Flavonoids | 7.0 |
| 12.2 | 935 | 633/301 | Castalagin/Vescalagin | Ellagitannins | 6.7 |
| 7.5 | 783.2 | 633/301 | Pedunculagin | Ellagitannins | 4.8 |
| 5.6 | 633.1 | 301/463 | Corilagin | Ellagitannins | 3.3 |
| 22.6 | 709.3 | 501/663 | 23-hydroxytormentic acid ester glucoside [M+HCOO]− isomer | Triterpenoids | 2.2 |
| 22.8 | 707.3 | 499/661 | Di-reduced 23-hydroxytormentic acid ester glucoside [M+HCOO]− | Triterpenoids | 1.8 |
| 25.6 | 503.2 | 485/471/453/441 | 23-hydroxytormentic acid | Triterpenoids | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zbeeb, H.; Baldini, F.; Zeaiter, L.; Vergani, L. The Anti-Inflammatory Potential of an Ethanolic Extract from Sarcopoterium spinosum Fruits for Protection and/or Counteraction against Oxidative Stress in Dysfunctional Endothelial Cells. Int. J. Mol. Sci. 2024, 25, 1601. https://doi.org/10.3390/ijms25031601
Zbeeb H, Baldini F, Zeaiter L, Vergani L. The Anti-Inflammatory Potential of an Ethanolic Extract from Sarcopoterium spinosum Fruits for Protection and/or Counteraction against Oxidative Stress in Dysfunctional Endothelial Cells. International Journal of Molecular Sciences. 2024; 25(3):1601. https://doi.org/10.3390/ijms25031601
Chicago/Turabian StyleZbeeb, Hawraa, Francesca Baldini, Lama Zeaiter, and Laura Vergani. 2024. "The Anti-Inflammatory Potential of an Ethanolic Extract from Sarcopoterium spinosum Fruits for Protection and/or Counteraction against Oxidative Stress in Dysfunctional Endothelial Cells" International Journal of Molecular Sciences 25, no. 3: 1601. https://doi.org/10.3390/ijms25031601