Multi-Targeting Neuroprotective Effects of Syzygium aromaticum Bud Extracts and Their Key Phytocompounds against Neurodegenerative Diseases
Abstract
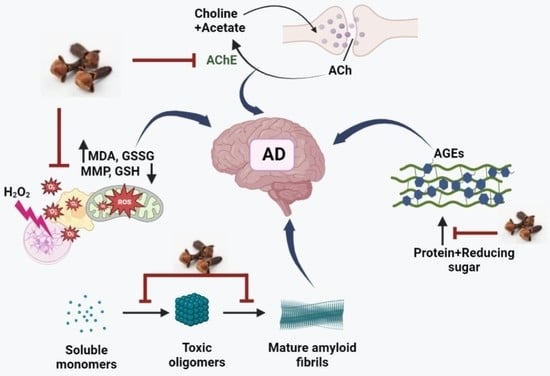
:1. Introduction
2. Results
2.1. Phytochemical Estimation and Antioxidant Potential of Clove Extract
2.2. GC–MS Analysis
2.3. In Vitro Anti-AGE Activity of Clove Extract
2.4. In Vitro Acetylcholinesterase Inhibitory Activity
2.5. Clove Extract Reduced Aβ Oligomerization and Fibrilization
2.6. Noncytotoxic Effect of Clove Extracts on the SH-SY5Y Cell Line
2.7. Clove Extracts Protected against H2O2-Induced Oxidative Stress
2.8. Clove Extracts Prevented H2O2-Induced ROS Generation
2.9. Restoration of Mitochondrial Membrane Potential by Clove Extract
2.10. Pre-Treatment of Clove Extract and Bioactive Compounds Restored Oxidative Stress Markers altered by H2O2-Induced Oxidative Stress
2.10.1. Restoration of Glutathione Levels
2.10.2. Attenuation of MDA Levels by Clove Extract
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Extraction
4.3. Gas Chromatography-Mass Spectrometry (GC-MS) Method
4.4. Determination of Total Phenolic Content
4.5. Determination of Total Flavonoids Content
4.6. Determination of Antioxidant Capacity
4.6.1. Free Radical Scavenging by 2,2-diphenyl-1-picrylhydrazylhydrate (DPPH) Radical
4.6.2. Free Radical Scavenging by 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic Acid) [ABTS] Radical
4.6.3. Ferric Reducing Antioxidant Potential (FRAP) Assay
4.7. Advanced Glycation End-Product (AGE) Inhibition Activity
4.8. Acetylcholinesterase Inhibitory Activity
4.9. Thioflavin T (ThT) Assay
4.10. Multiple Detection System (MDS)
4.11. Cell Culture
4.11.1. Cell Viability Assay
4.11.2. Neuroprotective Activity Assay
4.11.3. Measurement of Intracellular Reactive Oxygen Species (ROS)
4.11.4. Mitochondrial Membrane Potential (ΔΨm) Assay
4.11.5. Antioxidant Parameters in Cell Lysate
Protein Estimation
Estimation of Glutathione
Estimation of Malondialdehyde (MDA)
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Podtelezhnikov, A.A.; Tanis, K.Q.; Nebozhyn, M.; Ray, W.J.; Stone, D.J.; Loboda, A.P.; Loboda, A.P. Molecular insights into the pathogenesis of Alzheimer’s disease and its relationship to normal aging. PLoS ONE 2011, 6, e29610. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hamaguchi, T.; Naiki, H.; Yamada, M. Anti-amyloidogenic effects of antioxidants: Implications for the prevention and therapeutics of Alzheimer’s disease. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2006, 1762, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Giuffrida Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Tan, M.A.; An, S.S.A. Mechanistic aspects of Apiaceae family spices in ameliorating Alzheimer’s Disease. Antioxidants 2021, 10, 1571. [Google Scholar] [CrossRef]
- Tan, M.A.; Sharma, N.; An, S.S.A. Phyto-Carbazole Alkaloids from the Rutaceae Family as Potential Protective Agents against Neurodegenerative Diseases. Antioxidants 2022, 11, 493. [Google Scholar] [CrossRef]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. Molecular basis of the therapeutical potential of clove (Syzygium aromaticum L.) and clues to its anti-COVID-19 utility. Molecules 2021, 26, 1880. [Google Scholar] [CrossRef]
- Rosarior, V.L.; Lim, P.S.; Wong, W.K.; Yue, C.S.; Yam, H.C.; Tan, S.A. Antioxidant-rich Clove Extract, A Strong Antimicrobial Agent against Urinary Tract Infections-causing Bacteria in vitro. Trop. Life Sci. Res. 2021, 32, 45. [Google Scholar] [CrossRef]
- Tanko, Y.; Mohammed, A.; Okasha, M.A.; Umah, A.; Magaji, R. Anti-nociceptive and anti-inflammatory activities of ethanol extract of Syzygium aromaticum flower bud in wistar rats and mice. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 209–212. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Brebu, M.; Skalicka-Woźniak, K.; Luca, S.V. Phytochemical Characterization and Evaluation of the Antioxidant and Anti-Enzymatic Activity of Five Common Spices: Focus on Their Essential Oils and Spent Material Extractives. Plants 2021, 10, 2692. [Google Scholar] [CrossRef]
- DjidjouTagne, A.M.; Rosari, S.; Silalahi, R.L. Anti-Allergic Effect of Clove. Proceeding of 5th International Conference on Agro-Industry “Agroindustry 4.0—Digital Transformation on Agro-Food Value Chains”, Bali, Indonesia, 26–27 September 2018. [Google Scholar]
- Mejía-Argueta, E.; Santillán-Benítez, J.G.; Canales-Martinez, M.M.; Mendoza-Medellín, A. Antimicrobial activity of Syzygium aromaticum L. essential oil on extended-spectrum beta-lactamases-producing Escherichia coli. Bull. Natl. Res. Cent. 2020, 44, 201. [Google Scholar] [CrossRef]
- Nirmala, M.J.; Durai, L.; Gopakumar, V.; Nagarajan, R. Anticancer and antibacterial effects of a clove bud essential oil-based nanoscale emulsion system. Int. J. Nanomed. 2019, 14, 6439. [Google Scholar] [CrossRef]
- El-Hadary, A.E.; Ramadan Hassanien, M.F. Hepatoprotective effect of cold-pressed Syzygium aromaticum oil against carbon tetrachloride (CCl4)-induced hepatotoxicity in rats. Pharm. Biol. 2016, 54, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Mehta, A.K.; Kar, R.; Mustafa, M.; Mediratta, P.K.; Sharma, K.K. Clove oil reverses learning and memory deficits in scopolamine-treated mice. Planta Med. 2011, 77, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Panahzadeh, F.; Mirnasuri, R.; Rahmati, M. Exercise and Syzygium aromaticum reverse memory deficits, apoptosis and mitochondrial dysfunction of the hippocampus in Alzheimer’s disease. J. Ethnopharmacol. 2022, 286, 114871. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, F.; Mehrabi, S.; Naghizadeh, A.; Goudarzi, S. Comparison of intranasal and intraperitoneal administration of Eugenia caryophyllata (clove) essential oil on spatial memory, anxiety-like behavior and locomotor activity in a pilocarpine-induced status epilepticus rat model. BMC Complement. Med. Ther. 2022, 22, 231. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, K.Y.; Wang, Z.J.; Lu, Y.; Yin, M. N-stearoyl-l-Tyrosine inhibits the cell senescence and apoptosis induced by H2O2 in HEK293/Tau cells via the CB2 receptor. Chem.-Biol. Interact. 2017, 272, 135–144. [Google Scholar] [CrossRef]
- Somayajulu, M.; McCarthy, S.; Hung, M.; Sikorska, M.; Borowy-Borowski, H.; Pandey, S. Role of mitochondria in neuronal cell death induced by oxidative stress; neuroprotection by Coenzyme Q10. Neurobiol. Dis. 2005, 18, 618–627. [Google Scholar] [CrossRef]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of phenolic compounds in plant-defensive mechanisms. In Plant Phenolics in Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 517–532. [Google Scholar]
- Mathesius, U. Flavonoid functions in plants and their interactions with other organisms. Plants 2018, 7, 30. [Google Scholar] [CrossRef]
- González-Palma, I.; Escalona-Buendía, H.B.; Ponce-Alquicira, E.; Téllez-Téllez, M.; Gupta, V.K.; Díaz-Godínez, G.; Soriano-Santos, J. Evaluation of the antioxidant activity of aqueous and methanol extracts of Pleurotus ostreatus in different growth stages. Front. Microbiol. 2016, 7, 1099. [Google Scholar] [CrossRef]
- Lohani, U.C.; Fallahi, P.; Muthukumarappan, K. Comparison of ethyl acetate with hexane for oil extraction from various oilseeds. J. Am. Oil Chem. Soc. 2015, 92, 743–754. [Google Scholar] [CrossRef]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological properties and health benefits of eugenol: A comprehensive review. Oxidative Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- de Oliveira, M.S.; da Cruz, J.N.; Silva, S.G.; da Costa, W.A.; de Sousa, S.H.B.; Bezerra, F.W.F.; de Carvalho Junior, R.N. Phytochemical profile, antioxidant activity, inhibition of acetylcholinesterase and interaction mechanism of the major components of the Piper divaricatum essential oil obtained by supercritical CO2. J. Supercrit. Fluids 2019, 145, 74–84. [Google Scholar] [CrossRef]
- Shoaib, S.; Ansari, M.A.; Fatease, A.A.; Safhi, A.Y.; Hani, U.; Jahan, R.; Alomary, M.N.; Ansari, M.N.; Ahmed, N.; Wahab, S. Plant-Derived Bioactive Compounds in the Management of Neurodegenerative Disorders: Challenges, Future Directions and Molecular Mechanisms Involved in Neuroprotection. Pharmaceutics 2023, 15, 749. [Google Scholar] [CrossRef]
- Shim, K.H.; Sharma, N.; An, S.S.A. Mechanistic Insights into the Neuroprotective Potential of Sacred Ficus Trees. Nutrients 2022, 14, 4731. [Google Scholar] [CrossRef]
- Vastegani, S.M.; Khoshnam, S.E.; Mansouri, E.; Hajipour, S.; Ghafouri, S.; Bakhtiari, N.; Sarkaki, A.; Farbood, Y. Neuroprotective effect of anethole against rotenone induced non-motor deficits and oxidative stress in rat model of Parkinson’s disease. Behav. Brain Res. 2023, 437, 114100. [Google Scholar] [CrossRef]
- Iannuzzi, C.; Liccardo, M.; Sirangelo, I. Overview of the Role of Vanillin in Neurodegenerative Diseases and Neuropathophysiological Conditions. Int. J. Mol. Sci. 2023, 24, 1817. [Google Scholar] [CrossRef]
- Park, S.Y.; Jung, W.J.; Kang, J.S.; Kim, C.M.; Park, G.; Choi, Y.W. Neuroprotective effects of α-iso-cubebene against glutamate-induced damage in the HT22 hippocampal neuronal cell line. Int. J. Mol. Med. 2015, 35, 525–532. [Google Scholar] [CrossRef]
- Yokozawa, T.; Nakagawa, T. Inhibitory effects of Luobuma tea and its components against glucose-mediated protein damage. Food Chem. Toxicol. 2004, 42, 975–981. [Google Scholar] [CrossRef]
- Starowicz, M.; Zieliński, H. Inhibition of advanced glycation end-product formation by high antioxidant-leveled spices commonly used in European cuisine. Antioxidants 2019, 8, 100. [Google Scholar] [CrossRef]
- Perera, H.; Wijetunge, D. Strong protein glycation inhibitory potential of clove and coriander. Br. J. Pharm. Res. 2015, 6, 306–312. [Google Scholar] [CrossRef]
- Suantawee, T.; Wesarachanon, K.; Anantsuphasak, K.; Daenphetploy, T.; Thien-Ngern, S.; Thilavech, T.; Adisakwattana, S. Protein glycation inhibitory activity and antioxidant capacity of clove extract. J. Food Sci. Technol. 2015, 52, 3843–3850. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Jayaramaiah, R.H.; Agawane, S.B.; Vannuruswamy, G.; Korwar, A.M.; Anand, A.; Giri, A.P. Potential dual role of eugenol in inhibiting advanced glycation end products in diabetes: Proteomic and mechanistic insights. Sci. Rep. 2016, 6, 18798. [Google Scholar] [CrossRef] [PubMed]
- Rafey, A.; Amin, A.; Kamran, M.; Aziz, M.I.; Athar, V.; Niaz, S.I.; Pieters, L. Evaluation of Major Constituents of Medicinally Important Plants for Anti-Inflammatory, Antidiabetic and AGEs Inhibiting Properties: In Vitro and Simulatory Evidence. Molecules 2022, 27, 6715. [Google Scholar] [CrossRef]
- Balkrishna, A.; Pokhrel, S.; Tomer, M.; Verma, S.; Kumar, A.; Nain, P.; Gupta, A.; Varshney, A. Anti-acetylcholinesterase activities of mono-herbal extracts and exhibited synergistic effects of the phytoconstituents: A biochemical and computational study. Molecules 2019, 24, 4175. [Google Scholar] [CrossRef]
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G.; Serya, R.A. Anti-acetylcholinesterase activity of essential oils and their major constituents from four Ocimum species. Z. Für Nat. C 2016, 71, 393–402. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, V.; Singh, D. Kinetics of enzyme inhibition by active molluscicidal agents ferulic acid, umbelliferone, eugenol and limonene in the nervous tissue of snail Lymnaea acuminata. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 172–177. [Google Scholar]
- Tan, M.A.; Zakharova, E.; An, S.S.A. Diaportheone A Analogues Instigate a Neuroprotective Effect by Protecting Neuroblastoma SH-SY5Y Cells from Oxidative Stress. Biology 2021, 10, 199. [Google Scholar] [CrossRef]
- Kotormán, M.; Varga, A.; Kasi, P.B.; Nemcsók, J. Inhibition of the formation of amyloid-like fibrils with spices, especially cloves. Acta Biol. Hung. 2018, 69, 385–394. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Hamdi, D.; Ouachikh, O.; Ouchchane, L.; Omara-Reda, H.; Messaoud, C.; Hafidi, A. The neuroprotective effect of Clove essential oil against 6-OHDA-induced cell death in SH-SY5Y and a rat model of Parkinson’s disease. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Prasad, S.N.; Muralidhara. Neuroprotective efficacy of eugenol and isoeugenol in acrylamide-induced neuropathy in rats: Behavioral and biochemical evidence. Neurochem. Res. 2013, 38, 330–345. [Google Scholar] [CrossRef]
- Keramat, M.; Golmakani, M.T.; Durand, E.; Villeneuve, P.; Hosseini, S.M.H. jfpp A comparison of antioxidant activities by eugenyl acetate and eugenyl butyrate at frying temperature. J. Food Process. Preserv. 2021, 45, e15320. [Google Scholar] [CrossRef]
- Nogueira Sobrinho, A.C.; Morais, S.M.D.; Souza, E.B.D.; Albuquerque, M.R.J.R.; Santos, H.S.D.; Cavalcante, C.S.D.P.; Fontenelle, R.O.D.S. Antifungal and antioxidant activities of Vernonia chalybaea Mart. ex DC. essential oil and their major constituent β-caryophyllene. Braz. Arch. Biol. Technol. 2020, 63, e20190177. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Beserra, F.P.; Hussni, M.F.; Gonzaga, M.T.; Ribeiro, V.P.; De Souza, P.F.; Pellizzon, C.H. Beta-caryophyllene as an antioxidant, anti-inflammatory and re-epithelialization activities in a rat skin wound excision model. Oxid. Med. Cell. Longev. 2022, 2022, 9004014. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kikuchi, S.; Sasaki, N.; Suzuki, T.; Watai, T.; Iwaki, M.; Yamagishi, S.I. Involvement of advanced glycation end-products (AGEs) in Alzheimer’s disease. Curr. Alzheimer Res. 2004, 1, 39–46. [Google Scholar] [CrossRef]
- Maillard, L.C. Action des acides amines sur les sucres: Formation des melanoidines par voie methodique. CR Acad. Sci. 1912, 154, 66–68. [Google Scholar]
- Savateev, K.V.; Spasov, A.A.; Rusinov, V.L. Small synthetic molecules with antiglycation activity. Structure–activity relationship. Russ. Chem. Rev. 2022, 91, RCR5041. [Google Scholar] [CrossRef]
- Taha, M.; Alkadi, K.A.; Ismail, N.H.; Imran, S.; Adam, A.; Kashif, S.M.; Khan, K.M. Antiglycation and antioxidant potential of novel imidazo[4,5-b]pyridine benzohydrazones. Arab. J. Chem. 2019, 12, 3118–3128. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Gołąb, K.; Gburek, J.; Wysokińska, H.; Matkowski, A. Inhibition of Advanced Glycation End-Product Formation and Antioxidant Activity by Extracts and Polyphenols from Scutellaria alpina L. and S. altissima L. Molecules 2016, 21, 739. [Google Scholar] [CrossRef]
- Loh, Z.H.; Kwong, H.C.; Lam, K.W.; Teh, S.S.; Ee, G.C.L.; Quah, C.K.; Mah, S.H. New 3-O-substituted xanthone derivatives as promising acetylcholinesterase inhibitors. J. Enzym. Inhib. Med. Chem. 2021, 36, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Hung, N.H.; Quan, P.M.; Satyal, P.; Dai, D.N.; Hoa, V.V.; Huy, N.G.; Giang, L.D.; Ha, N.T.; Huong, L.T.; Hien, V.T.; et al. N. Acetylcholinesterase Inhibitory Activities of Essential Oils from Vietnamese Traditional Medicinal Plants. Molecules 2022, 27, 7092. [Google Scholar] [CrossRef] [PubMed]
- Zamli, K.M.; Asari, A.; Khaw, K.Y.; Murugaiyah, V.; Al-Rashida, H.M.; Yusoff, H.M.; Hasnah, N.H.A.W. Cholinesterase Inhibition Activity and Molecular Docking Study of Eugenol Derivatives. Sains Malays. 2021, 50, 1037–1045. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V. Beneficial effects of walnuts on cognition and brain health. Nutrients 2020, 12, 550. [Google Scholar] [CrossRef]
- Gazit, E. Mechanisms of amyloid fibril self-assembly and inhibition: Model short peptides as a key research tool. FEBS J. 2005, 272, 5971–5978. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of eugenol: A structure–activity relationship study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef]
- Gaspar, E.M.; Duarte, R.; Santana, J.C. Volatile composition and antioxidant properties of clove products. Biomed. J. Sci. Tech. Res. 2018, 9, 7270–7276. [Google Scholar] [CrossRef]
- Ullah, H.; Di Minno, A.; Santarcangelo, C.; Khan, H.; Daglia, M. Improvement of oxidative stress and mitochondrial dysfunction by β-caryophyllene: A focus on the nervous system. Antioxidants 2021, 10, 546. [Google Scholar] [CrossRef]
- Shekhar, S.; Yadav, Y.; Singh, A.P.; Pradhan, R.; Desai, G.R.; Dey, A.B.; Dey, S. Neuroprotection by ethanolic extract of Syzygium aromaticum in Alzheimer’s disease like pathology via maintaining oxidative balance through SIRT1 pathway. Exp. Gerontol. 2018, 110, 277–283. [Google Scholar] [CrossRef]
- Amir Rawa, M.S.; Mazlan, M.K.N.; Ahmad, R.; Nogawa, T.; Wahab, H.A. Roles of Syzygium in Anti-Cholinesterase, Anti-Diabetic, Anti-Inflammatory, and Antioxidant: From Alzheimer’s Perspective. Plants 2022, 11, 1476. [Google Scholar] [CrossRef]
- Latif, S.; Choi, S.-H.; Gyawali, A.; Hyeon, S.J.; Kang, Y.-S.; Ryu, H. Antioxidant and Neuroprotective Effects of Paeonol against Oxidative Stress and Altered Carrier-Mediated Transport System on NSC-34 Cell Lines. Antioxidants 2022, 11, 1392. [Google Scholar] [CrossRef]
- Adki, K.M.; Kulkarni, Y.A. Neuroprotective effect of paeonol in streptozotocin-induced diabetes in rats. Life Sci. 2021, 271, 119202. [Google Scholar] [CrossRef]
- Usta, J.; Kreydiyyeh, S.; Bajakian, K.; Nakkash-Chmaisse, H. In vitro effect of eugenol and cinnamaldehyde on membrane potential and respiratory chain complexes in isolated rat liver mitochondria. Food Chem. Toxicol. 2002, 40, 935–940. [Google Scholar] [CrossRef]
- Zhang, Z.-D.; Yang, Y.J.; Liu, X.W.; Qin, Z.; Li, S.H.; Bai, L.X.; Li, J.Y. The Protective Effect of Aspirin Eugenol Ester on Oxidative Stress to PC12 Cells Stimulated with H2O2 through Regulating PI3K/Akt Signal Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 5527475. [Google Scholar] [CrossRef]
- Mesole, S.B.; Alfred, O.O.; Yusuf, U.A.; Lukubi, L.; Ndhlovu, D. Apoptotic inducement of neuronal cells by aluminium chloride and the neuroprotective effect of eugenol in wistar rats. Oxid. Med. Cell. Longev. 2020, 2020, 8425643. [Google Scholar] [CrossRef]
- Assis, L.; Straliotto, M.R.; Engel, D.; Hort, M.A.; Dutra, R.C.; De Bem, A.F. β-Caryophyllene protects the C6 glioma cells against glutamate-induced excitotoxicity through the Nrf2 pathway. Neuroscience 2014, 279, 220–231. [Google Scholar] [CrossRef]
- Wang, G.; Ma, W.; Du, J. β-Caryophyllene (BCP) ameliorates MPP+ induced cytotoxicity. Biomed. Pharmacother. 2018, 103, 1086–1091. [Google Scholar] [CrossRef]
- Tayanloo-Beik, A.; Kiasalari, Z.; Roghani, M. Paeonol Ameliorates Cognitive Deficits in Streptozotocin Murine Model of Sporadic Alzheimer’s Disease via Attenuation of Oxidative Stress, Inflammation, and Mitochondrial Dysfunction. J. Mol. Neurosci. 2022, 72, 336–348. [Google Scholar] [CrossRef]
- Boadi, W.Y.; Stevenson, C.; Johnson, D.; Mohamed, M.A. Flavonoids reduce lipid peroxides and increase glutathione levels in pooled human liver microsomes (HLMs). Adv. Biol. Chem. 2021, 11, 283–295. [Google Scholar] [CrossRef]
- Kim, S.H.; Smith, A.J.; Tan, J.; Shytle, R.D.; Giunta, B. MSM ameliorates HIV-1 Tat induced neuronal oxidative stress via rebalance of the glutathione cycle. Am. J. Transl. Res. 2015, 7, 328–338. [Google Scholar] [PubMed]
- Verma, A.R.; Vijayakumar, M.; Rao, C.V.; Mathela, C.S. In vitro and in vivo antioxidant properties and DNA damage protective activity of green fruit of Ficus glomerata. Food Chem. Toxicol. 2010, 48, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A.; ur Rahman, U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential oil eugenol: Sources, extraction techniques and nutraceutical perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef]
- Adegbola, M.V.; Anyim, G.; Ntwasa, M.; Ayeleso, A.O.; Oyedepo, T.A. Potential Effect of Syzygium aromaticum (Cloves) Extract on Serum Antioxidant Status and Lipid Profiles in Wistar Rats with Artesunate Toxicity. Appl. Sci. 2022, 12, 8216. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Meth. Enzymol.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Ribarova, F.; Atanassova, M.; Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. JU Chem. Metal 2005, 40, 255–260. [Google Scholar]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.; Groot, A.D.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem. Toxicol. 2013, 55, 290–296. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- An, S.S.A.; Shim, K.H.; Kang, S.; Kim, Y.K.; Subedi, L.; Cho, H.; Kim, S. The potential anti-amyloidogenic candidate, SPA1413, for Alzheimer’s disease. Br. J. Pharmacol. 2022, 179, 1033–1048. [Google Scholar] [CrossRef]
- PeÑalver, P.; Zodio, S.; Lucas, R.; de-Paz, M.V.; Morales, J.C. Neuroprotective and anti-inflammatory effects of pterostilbene metabolites in human neuroblastoma SH-SY5Y and RAW 264.7 macrophage cells. J. Agric. Food Chem. 2020, 68, 1609–1620. [Google Scholar] [CrossRef]
- Alvariño, R.; Alonso, E.; Lacret, R.; Oves-Costales, D.; Genilloud, O.; Reyes, F.; Botana, L.M. Caniferolide A, a macrolide from Streptomyces caniferus, attenuates neuroinflammation, oxidative stress, amyloid-beta, and tau pathology in vitro. Mol. Pharm. 2019, 16, 1456–1466. [Google Scholar] [CrossRef]
- Harlow, E.; Lane, D. Lysing tissue-culture cells for immunoprecipitation. Cold Spring Harb. Protoc. 2006, 2006, pdb.prot4531. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Singh, V.; Gera, R.; Purohit, M.P.; Patnaik, S.; Ghosh, D. Fluorometric estimation of glutathione in cultured microglial cell lysate. Bio-Protoc. 2017, 7, e2304. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]













| Vmax (μmole/min/mg) | Km (mM) | Type of Inhibition | |
|---|---|---|---|
| No Inhibitor | 3.90 ± 0.09 | 10.49 ± 0.02 | |
| CL-H | 3.90 ±0.17 | 17.64 ± 0.04 | Competitive |
| CL-EA | 4.24 ± 0.06 | 12.28 ±0.01 | Competitive |
| Eugenol | 4.01 ± 0.14 | 14.19 ± 0.03 | Competitive |
| β-Caryophyllene | 4.21 ± 0.19 | 17.87 ± 0.05 | Competitive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, H.; Kim, D.Y.; Shim, K.H.; Sharma, N.; An, S.S.A. Multi-Targeting Neuroprotective Effects of Syzygium aromaticum Bud Extracts and Their Key Phytocompounds against Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 8148. https://doi.org/10.3390/ijms24098148
Sharma H, Kim DY, Shim KH, Sharma N, An SSA. Multi-Targeting Neuroprotective Effects of Syzygium aromaticum Bud Extracts and Their Key Phytocompounds against Neurodegenerative Diseases. International Journal of Molecular Sciences. 2023; 24(9):8148. https://doi.org/10.3390/ijms24098148
Chicago/Turabian StyleSharma, Himadri, Dan Yeong Kim, Kyu Hwan Shim, Niti Sharma, and Seong Soo A. An. 2023. "Multi-Targeting Neuroprotective Effects of Syzygium aromaticum Bud Extracts and Their Key Phytocompounds against Neurodegenerative Diseases" International Journal of Molecular Sciences 24, no. 9: 8148. https://doi.org/10.3390/ijms24098148