Confirmation of Statin and Fibrate Use from Small-Volume Archived Plasma Samples by High-Throughput LC-MS/MS Method
Abstract
:1. Introduction
2. Results
2.1. Optimization of LC-MS/MS Conditions
2.2. Selection of Protein Precipitation Solvent and Recovery
2.3. Determination of Detection Criteria
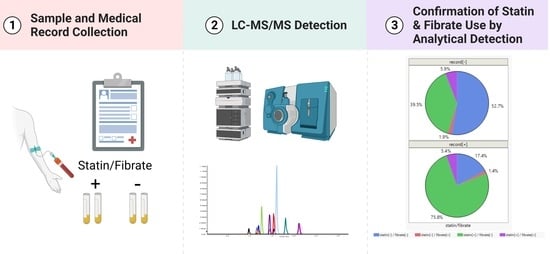
2.4. Application
3. Discussion
3.1. Comparing Sensitivity and Accuracy of Our Method with Existing Methods
3.2. Confidence in the Reported Number of Detects
3.3. Accuracy of Statin Use Information Based on Self-Reported Records
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Collection
4.3. Calibrators, Quality Control Samples, and Internal Standards and Solution
4.4. Sample Preparation
4.5. Liquid Chromatography–Mass Spectrometry Method
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- World Health Organization. Cardiovascular Diseases (Cvds). 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 31 May 2022).
- Lloyd-Jones, D.M.; LBraun, T.; Ndumele, C.E.; Smith, S.C.; Sperling, L.S.; Virani, S.S.; Blumenthal, R.S. Use of risk assessment tools to guide decision-making in the primary prevention of atherosclerotic cardiovascular disease. J. Am. Coll. Cardiol. 2019, 73, 3153–3167. [Google Scholar] [CrossRef]
- Austin, M.A.; Hutter, C.M.; Zimmern, R.L.; Humphries, S.E. Genetic causes of monogenic heterozygous familial hypercholesterolemia: A huge prevalence review. Am. J. Epidemiol. 2004, 160, 407–420. [Google Scholar] [CrossRef]
- Wang, T.Y.; Newby, L.K.; Chen, A.Y.; Mulgund, J.; Roe, M.T.; Sonel, A.F.; Bhatt, D.L.; DeLong, E.R.; Ohman, E.M.; Gibler, W.B.; et al. Hypercholesterolemia paradox in relation to mortality in acute coronary syndrome. Clin. Cardiol. 2009, 32, E22–E28. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Won, H.-H.; Peloso, G.M.; Lawson, K.S.; Bartz, T.M.; Deng, X.; van Leeuwen, E.M.; Natarajan, P.; Emdin, C.A.; Bick, A.G.; et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J. Am. Coll. Cardiol. 2016, 67, 2578–2589. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the european atherosclerosis society consensus panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Shitara, Y.; Sugiyama, Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme a (hmg-coa) reductase inhibitors: Drug–drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol. Ther. 2006, 112, 71–105. [Google Scholar] [CrossRef]
- Gu, Q.; Paulose-Ram, R.; Burt, V.L.; Kit, B.K. Prescription Cholesterol-Lowering Medication Use in Adults Aged 40 and Over: United States, 2003–2012; National Center for Health Statistics: Hyattsville, MD, USA, 2014. [Google Scholar]
- Jones, P.H. Chapter 26—Fibrates. In Clinical Lipidology; Ballantyne, C.M., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2009; pp. 315–325. [Google Scholar]
- Welty, F.K. How do elevated triglycerides and low hdl-cholesterol affect inflammation and atherothrombosis? Curr. Cardiol. Rep. 2013, 15, 400. [Google Scholar] [CrossRef]
- Sandesara, P.B.; Virani, S.S.; Fazio, S.; Shapiro, M.D. The forgotten lipids: Triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr. Rev. 2019, 40, 537–557. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Zimmerman, F.H. Fibrates in combination with statins in the management of dyslipidemia. J. Clin. Hypertens. 2006, 8, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.M.; Bryson, C.L.; Rumsfeld, J.S. Medication adherence. Circulation 2009, 119, 3028–3035. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, C.; Kousoulos, C.; Dotsikas, Y.; Soumelas, G.-S.; Kolocouri, F.; Ziaka, A.; Loukas, Y.L. An improved and fully validated lc–ms/ms method for the simultaneous quantification of simvastatin and simvastatin acid in human plasma. J. Pharm. Biomed. Anal. 2008, 46, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Dias, E.; Hachey, B.; McNaughton, C.; Nian, H.; Yu, C.; Straka, B.; Brown, N.J.; Caprioli, R.M. An lc–ms assay for the screening of cardiovascular medications in human samples. J. Chromatogr. B 2013, 937, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.K.; Martin, P.D.; Warwick, M.J.; Thomas, E. Quantification of the n-desmethyl metabolite of rosuvastatin in human plasma by automated spe followed by hplc with tandem ms detection. J. Pharm. Biomed. Anal. 2004, 35, 609–614. [Google Scholar] [CrossRef]
- Hull, C.K.; Penman, A.D.; Smith, C.K.; Martin, P.D. Quantification of rosuvastatin in human plasma by automated solid-phase extraction using tandem mass spectrometric detection. J. Chromatogr. B 2002, 772, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Mertens, B.; Cahay, B.; Klinkenberg, R.; Streel, B. An automated method for the simultaneous determination of pravastatin, 3-hydroxy isomeric metabolite, pravalactone and fenofibric acid in human plasma by sensitive liquid chromatography combined with diode array and tandem mass spectrometry detection. J. Chromatogr. A 2008, 1189, 493–502. [Google Scholar] [CrossRef]
- Nirogi, R.V.S.; VKandikere, N.; Shrivastava, W.; Mudigonda, K.; Datla, P.V. Liquid chromatography/negative ion electrospray tandem mass spectrometry method for the quantification of fluvastatin in human plasma: Validation and its application to pharmacokinetic studies. Rapid Commun. Mass Spectrom. 2006, 20, 1225–1230. [Google Scholar] [CrossRef]
- Patel, B.N.; Sharma, N.; Sanyal, M.; Shrivastav, P.S. Simultaneous determination of simvastatin and simvastatin acid in human plasma by lc-ms/ms without polarity switch: Application to a bioequivalence study. J. Sep. Sci. 2008, 31, 301–313. [Google Scholar] [CrossRef]
- Roadcap, B.A.; Musson, D.G.; Rogers, J.D.; Zhao, J.J. Sensitive method for the quantitative determination of gemfibrozil in dog plasma by liquid–liquid cartridge extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2003, 791, 161–170. [Google Scholar] [CrossRef]
- Shen-Tu, J.; Xu, X.; Liu, J.; Hu, X.; Chen, J.; Wu, L.; Huang, M.; Zhou, H. Determination of pitavastatin in human plasma by lc–ms–ms. Chromatographia 2009, 69, 1041–1047. [Google Scholar] [CrossRef]
- Trivedi, R.K.; Kallem, R.R.; Mullangi, R.; Srinivas, N.R. Simultaneous determination of rosuvastatin and fenofibric acid in human plasma by lc–ms/ms with electrospray ionization: Assay development, validation and application to a clinical study. J. Pharm. Biomed. Anal. 2005, 39, 661–669. [Google Scholar] [CrossRef]
- Wagmann, L.; Hemmer, S.; Caspar, A.T.; Meyer, M.R. Method development for quantitative determination of seven statins including four active metabolites by means of high-resolution tandem mass spectrometry applicable for adherence testing and therapeutic drug monitoring. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 664–672. [Google Scholar] [CrossRef]
- Wang, J.; Luzum, J.A.; Phelps, M.A.; Kitzmiller, J.P. Liquid chromatography–tandem mass spectrometry assay for the simultaneous quantification of simvastatin, lovastatin, atorvastatin, and their major metabolites in human plasma. J. Chromatogr. B 2015, 983–984, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, H.; Uchiyama, N.; Imagaki, K.; Hata, S.; Kamei, T. Determination of simvastatin and its active metabolite in human plasma by column-switching high-performance liquid chromatography with fluorescence detection after derivatization with 1-bromoacetylpyrene. J. Chromatogr. B Biomed. Sci. Appl. 1997, 694, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Busse, K.H.; Hadigan, C.; Chairez, C.; Alfaro, R.M.; Formentini, E.; Kovacs, J.A.; Penzak, S.R. Gemfibrozil concentrations are significantly decreased in the presence of lopinavir-ritonavir. J. Acquir. Immune Defic. Syndr. 2009, 52, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.B.; Spence, J.D. Clinical pharmacokinetics of fibric acid derivatives (fibrates). Clin. Pharmacokinet. 1998, 34, 155–162. [Google Scholar] [CrossRef]
- Rosenson, R.S. Rosuvastatin: A new inhibitor of hmg-coa reductase for the treatment of dyslipidemia. Expert Rev. Cardiovasc. Ther. 2003, 1, 495–505. [Google Scholar] [CrossRef]
- Singhvi, S.M.; Pan, H.Y.; Morrison, R.A.; Willard, D.A. Disposition of pravastatin sodium, a tissue-selective hmg-coa reductase inhibitor, in healthy subjects. Br. J. Clin. Pharmacol. 1990, 29, 239–243. [Google Scholar] [CrossRef]
- Qin, X.-Z. Collision-induced dissociation of the negative ions of simvastatin hydroxy acid and related species. J. Mass Spectrom. 2003, 38, 677–686. [Google Scholar] [CrossRef]
- Shandilya, D.; Israni, R.; Joseph, P. Prediction of the fragmentation pathway of atorvastatin de-protonated ion. OALib 2018, 5, 1–14. [Google Scholar] [CrossRef]



| Target | Type of the Target | Precursor Mass (Da) | Product Mass (Da) | DP (V) | CE (V) | CXP (V) | RT (min) |
|---|---|---|---|---|---|---|---|
| 2-OH-Atorvastatin | Metabolite of Atorvastatin | 573.2 | 278.0 | −73 | −39 | −33 | 1.38 |
| 2-OH-Atorvastatin-d5 | Internal Standard | 578.3 | 283.1 | −73 | −39 | −33 | 1.38 |
| 4-OH-Atorvastatin | Metabolite of Atorvastatin | 573.2 | 413.1 | −73 | −39 | −33 | 1.09 |
| 4-OH-Atorvastatin-d5 | Internal Standard | 578.2 | 418.2 | −73 | −39 | −33 | 1.09 |
| Atorvastatin | Parent Drug | 557.2 | 278.1 | −155 | −60 | −5 | 1.48 |
| Atorvastatin-d5 | Internal Standard | 562.1 | 283.2 | −155 | −60 | −5 | 1.47 |
| Clofibric Acid | Metabolite of Clofibrate | 213.0 | 127.0 | −40 | −25 | −8.4 | 1.21 |
| Clofibric Acid-d4 | Internal Standard | 217.1 | 131.0 | −40 | −25 | −8.4 | 1.19 |
| Fenofibric Acid | Metabolite of Fenofibrate | 317.1 | 231.0 | −50 | −37 | −15.6 | 1.56 |
| Fenofibric Acid-d5 | Internal Standard | 323.1 | 231.0 | −50 | −37 | −15.6 | 1.55 |
| Fluvastatin | Parent Drug | 410.3 | 348.2 | −22 | −21 | −25 | 1.48 |
| Fluvastatin-d6 | Internal Standard | 416.2 | 354.2 | −22 | −21 | −25 | 1.48 |
| Gemfibrozil | Parent Drug | 249.0 | 121.1 | −24 | −23 | −10 | 2.09 |
| Gemfibrozil-d6 | Internal Standard | 255.2 | 121.1 | −24 | −23 | −10 | 2.08 |
| Lovastatin Acid | Metabolite of Lovastatin | 421.4 | 101.0 | −72 | −27 | −10 | 1.75 |
| Lovastatin Acid-d3 | Internal Standard | 424.3 | 104.1 | −72 | −27 | −10 | 1.73 |
| N-Desmethyl-Rosuvastatin * | Metabolite of Rosuvastatin | 466.2 | 404.1 | −28 | −23 | −27 | 0.85 |
| Pitavastatin | Parent Drug | 420.2 | 358.1 | −40 | −18 | −16 | 1.13 |
| Pitavastatin-d5 | Internal Standard | 425.2 | 363.1 | −40 | −18 | −16 | 1.12 |
| Pravastatin | Parent Drug | 423.4 | 321.2 | −80 | −23 | −51 | 0.92 |
| Pravastatin-d3 | Internal Standard | 426.2 | 321.2 | −80 | −23 | −51 | 0.91 |
| Rosuvastatin | Parent Drug | 479.8 | 418.3 | −34 | −17 | −23 | 1.13 |
| Rosuvastatin-d3 | Internal Standard | 483.2 | 421.2 | −34 | −17 | −23 | 1.12 |
| Simvastatin Acid | Metabolite of Simvastatin | 434.9 | 319.2 | −118 | −21 | −21.4 | 2.09 |
| Simvastatin Acid-d6 | Internal Standard | 441.4 | 319.2 | −118 | −21 | −21.4 | 2.05 |
| Spiking Levels (ng/mL) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1× LLOD | 2× LLOD | 5× LLOD | |||||||||||
| Analytes | LLOD (ng/mL) | Intra-Day (n = 18) | Inter-Day (n = 24) | Intra-Day (n = 18) | Inter-Day (n = 24) | Intra-Day (n = 18) | Inter-Day (n = 24) | ||||||
| %Acc | %CV | %CV | ΔRT | %Acc | %CV | %CV | ΔRT | %Acc | %CV | %CV | ΔRT | ||
| 2-OH-Atorvastatin | 0.05 | 106 | 26 | 36 | 0.026 | 106 | 13 | 36 | 0.025 | 96 | 8 | 33 | 0.040 |
| 4-OH-Atorvastatin | 0.5 | 84 | 13 | 20 | 0.019 | 101 | 11 | 20 | 0.018 | 107 | 13 | 29 | 0.017 |
| Atorvastatin | 0.1 | 97 | 25 | 37 | 0.058 | 94 | 26 | 37 | 0.037 | 88 | 18 | 30 | 0.043 |
| Clofibric Acid | 0.05 | 140 | 37 | 38 | 0.064 | 102 | 22 | 38 | 0.069 | 100 | 19 | 23 | 0.073 |
| Fenofibric Acid | 0.05 | 127 | 31 | 25 | 0.046 | 120 | 20 | 25 | 0.044 | 113 | 10 | 21 | 0.140 |
| Fluvastatin | 0.5 | 117 | 13 | 21 | 0.022 | 110 | 12 | 21 | 0.016 | 97 | 9 | 15 | 0.018 |
| Gemfibrozil | 0.5 | 119 | 5 | 34 | 0.019 | 113 | 4 | 34 | 0.019 | 105 | 7 | 9 | 0.017 |
| Lovastatin Acid | 0.5 | 99 | 13 | 33 | 0.024 | 98 | 15 | 33 | 0.024 | 97 | 7 | 22 | 0.023 |
| Pitavastatin | 0.5 | 117 | 30 | 29 | 0.013 | 108 | 27 | 29 | 0.016 | 104 | 7 | 17 | 0.018 |
| Pravastatin | 0.5 | 84 | 13 | 44 | 0.015 | 95 | 12 | 44 | 0.013 | 101 | 8 | 9 | 0.010 |
| Rosuvastatin | 0.5 | 140 | 29 | 47 | 0.027 | 105 | 28 | 47 | 0.027 | 113 | 14 | 24 | 0.024 |
| Simvastatin Acid | 0.5 | 100 | 11 | 37 | 0.030 | 106 | 5 | 37 | 0.019 | 104 | 6 | 14 | 0.026 |
| Recorded Medication | Ator | Sim | Pra | Lov | Rosu | Flu | Sim/Eze | Statin-Record [+] | Statin-Record [−] |
|---|---|---|---|---|---|---|---|---|---|
| Medication Statin Record | 174 | 105 | 33 | 12 | 11 | 3 | 5 | Σ351 | 590 |
| (% Statin-Record [+]/Σ941 | (19.0) | (11.4) | (3.6) | (1.3) | (1.2) | (0.3) | (0.5) | (37.3) | (62.7) |
| % of Statin-Record [+]/Σ351 | 49.6 | 29.9 | 9.4 | 3.4 | 3.1 | 0.9 | 1.4 | ||
| Analyzed Statins | Number of samples with recorded statin medication | Σ Statin-Detect [+] in Statin-Record [+] | Σ Statin-Detect [+] in Statin-Record [−] | ||||||
| Atorvastatin [+] | 155 | 9 | 3 | 2 | 0 | 0 | 1 | 170 | 176 |
| Simvastatin [+] | 1 | 61 | 1 | 0 | 1 | 0 | 2 | 66 | 46 |
| Pravastatin [+] | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 20 | 5 |
| Lovastatin [+] | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 8 | 6 |
| Rosuvastatin [+] | 0 | 2 | 0 | 0 | 6 | 0 | 0 | 8 | 15 |
| Fluvastatin [+] | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 4 | 2 |
| Pitavastatin [+] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Σ Statin Detect [+] | Σ157/174 | Σ73/105 | Σ24/33 | Σ10/12 | Σ7/11 | Σ2/3 | Σ3/5 | Σ276/Σ351 | Σ250/590 |
| % Statin Detect [+] confirmed | 90.2 | 69.5 | 72.7 | 83.3 | 63.6 | 66.7 | 60.0 | 78.6 | 42.4 |
| Medication confirmed | 155/174 | 61/105 | 20/33 | 8/12 | 6/11 | 2/3 | 2/5 | Σ255/351 | |
| % Medication confirmed | 89.1 | 58.1 | 60.6 | 66.7 | 54.5 | 66.7 | 40.0 | 72.4 | |
| Analyzed Fibrates | Number of samples with recorded statin medication | Σ Fibrate-Detect [+] in Statin-Record [+] | Σ Fibrate-Detect [+] in Statin-Record [−] | ||||||
| Clofibrate [+] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fenofibrate [+] | 9 | 4 | 1 | 0 | 0 | 0 | 0 | 14 | 23 |
| Gemfibrozil [+] | 6 | 2 | 2 | 0 | 0 | 0 | 0 | 10 | 23 |
| Σ Fibrate Detect [+] | Σ15/174 | Σ6/105 | Σ3/33 | 0 | 0 | 0 | 0 | Σ24/Σ351 | Σ46/590 |
| % Fibrate Detect [+] | 8.6 | 5.7 | 9.1 | 0 | 0 | 0 | 0 | 6.8 | 7.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusovschi, J.D.; Ivanova, A.A.; Gardner, M.S.; McGarrah, R.W., III; Kraus, W.E.; Kuklenyik, Z.; Pirkle, J.L.; Barr, J.R. Confirmation of Statin and Fibrate Use from Small-Volume Archived Plasma Samples by High-Throughput LC-MS/MS Method. Int. J. Mol. Sci. 2023, 24, 7931. https://doi.org/10.3390/ijms24097931
Kusovschi JD, Ivanova AA, Gardner MS, McGarrah RW III, Kraus WE, Kuklenyik Z, Pirkle JL, Barr JR. Confirmation of Statin and Fibrate Use from Small-Volume Archived Plasma Samples by High-Throughput LC-MS/MS Method. International Journal of Molecular Sciences. 2023; 24(9):7931. https://doi.org/10.3390/ijms24097931
Chicago/Turabian StyleKusovschi, Jennifer D., Anna A. Ivanova, Michael S. Gardner, Robert W. McGarrah, III, William E. Kraus, Zsuzsanna Kuklenyik, James L. Pirkle, and John R. Barr. 2023. "Confirmation of Statin and Fibrate Use from Small-Volume Archived Plasma Samples by High-Throughput LC-MS/MS Method" International Journal of Molecular Sciences 24, no. 9: 7931. https://doi.org/10.3390/ijms24097931