Interaction between Six Waxy Components in Summer Black Grapes (Vitis vinifera) and Mancozeb and Its Effect on the Residue of Mancozeb
Abstract
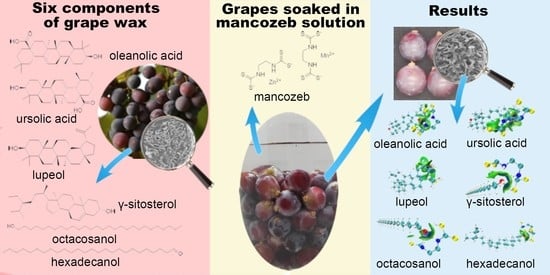
:1. Introduction
2. Results and Discussion
2.1. Effect of Solvent Extraction on the Wax Layer
2.2. Waxy Layer Composition Analysis
2.3. Molecular Docking Simulation and IGMH Analysis between Mancozeb and Waxy Components
2.4. Comparison of Mancozeb Content in Grapes
3. Materials and Methods
3.1. Grape Materials, Chemicals, and Standards
3.2. Electron Microscopy of Grape Peel
3.3. Wax Content Determination
3.4. Wax Analysis
3.5. Simulation Analysis of the Combination of Pesticides and Waxes
3.6. Detection of Pesticide Residues in Grapes
3.7. Data Analysis and Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roca, P.; Di Lorenzo, C.; Colombo, F.; Sangiovanni, E.; Biella, S.; Regazzoni, L.; Peres de Sousa, L.; Bavaresco, L.; Tomasi, D.; Bosso, A.; et al. Phenolic profile and biological activity of table grapes (Vitis vinifera L.). BIO Web Conf. 2019, 15, 04005. [Google Scholar] [CrossRef]
- Garcia-Coronado, H.; Tafolla-Arellano, J.C.; Hernandez-Onate, M.A.; Burgara-Estrella, A.J.; Robles-Parra, J.M.; Tiznado-Hernandez, M.E. Molecular biology, composition and physiological functions of cuticle lipids in fleshy fruits. Plants 2022, 11, 1133. [Google Scholar] [CrossRef] [PubMed]
- Szakiel, A.; Paczkowski, C.; Pensec, F.; Bertsch, C. Fruit cuticular waxes as a source of biologically active triterpenoids. Phytochem. Rev. 2012, 11, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Casado, C.G.; Heredia, A. Structure and dynamics of reconstituted cuticular waxes of grape berry cuticle (Vitis vinifera L.). J. Exp. Bot. 1999, 50, 175–182. [Google Scholar] [CrossRef]
- Wang, J.; Hao, H.; Liu, R.; Ma, Q.; Xu, J.; Chen, F.; Cheng, Y.; Deng, X. Comparative analysis of surface wax in mature fruits between Satsuma mandarin (Citrus unshiu) and ‘Newhall’ navel orange (Citrus sinensis) from the perspective of crystal morphology, chemical composition and key gene expression. Food Chem. 2014, 153, 177–185. [Google Scholar] [CrossRef]
- Arand, K.; Bieler, E.; Durrenberger, M.; Kassemeyer, H.H. Developmental pattern of grapevine (Vitis vinifera L.) berry cuticular wax: Differentiation between epicuticular crystals and underlying wax. PLoS ONE 2021, 16, e0246693. [Google Scholar] [CrossRef]
- Trivedi, P.; Nguyen, N.; Hykkerud, A.L.; Haggman, H.; Martinussen, I.; Jaakola, L.; Karppinen, K. Developmental and environmental regulation of cuticular wax biosynthesis in fleshy fruits. Front. Plant. Sci. 2019, 10, 431–440. [Google Scholar] [CrossRef]
- Hwang, J.-I.; Seok, D.-R.; Kim, J.-E. Effects of cuticular waxes on permeation of fungicides azoxystrobin and chlorothalonil into apples. Appl. Biol. Chem. 2019, 62, 34. [Google Scholar] [CrossRef]
- Staiger, S.; Seufert, P.; Arand, K.; Burghardt, M.; Popp, C.; Riederer, M. The permeation barrier of plant cuticles: Uptake of active ingredients is limited by very long-chain aliphatic rather than cyclic wax compounds. Pest. Manag. Sci. 2019, 75, 3405–3412. [Google Scholar] [CrossRef]
- Hwang, E.S.; Cash, J.N.; Zabik, M.J. Degradation of mancozeb and ethylenethiourea in apples due to postharvest treatments and processing. J. Food Sci. 2002, 67, 3295–3300. [Google Scholar] [CrossRef]
- Thind, T.S.; Hollomon, D.W. Thiocarbamate fungicides: Reliable tools in resistance management and future outlook. Pest. Manag. Sci. 2018, 74, 1547–1551. [Google Scholar] [CrossRef]
- Kumar, Y.B.; Shabeer, T.P.A.; Jadhav, M.; Banerjee, K.; Hingmire, S.; Saha, S.; Rai, A.B. Analytical method validation, dissipation and safety evaluation of combination fungicides fenamidone + mancozeb and iprovalicarb + propineb in/on tomato. J. Food Sci. Technol. 2020, 57, 2061–2069. [Google Scholar] [CrossRef]
- Qi, G.; Xinghong, L.; Mei, L. Investigation on occurrence and damge of vineyard diseases and control pesticides in China. China Fruits 2021, 9, 97–102. [Google Scholar] [CrossRef]
- Lopez-Fernandez, O.; Pose-Juan, E.; Rial-Otero, R.; Simal-Gandara, J. Effects of hydrochemistry variables on the half-life of mancozeb and on the hazard index associated to the sum of mancozeb and ethylenethiourea. Environ. Res. 2017, 154, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-J.; Yin, C.-M.; Wang, Y.; Kong, Y.-H.; Wang, G.-H.; Wu, C.-Y.; Zhang, L.-F.; Liu, Z.-R. The thermal behaviour of the ethylenebisdithiocarbamates Maneb, Zineb and Mancozeb.: Part 1. The mechanism of the thermal decomposition. Thermochim. Acta 1993, 220, 213–227. [Google Scholar] [CrossRef]
- Dall’Agnol, J.C.; Pezzini, M.F.; Uribe, N.S.; Joveleviths, D. Systemic effects of the pesticide mancozeb—A literature review. Eur. Rev. Med. Pharmaco 2021, 25, 4113–4120. [Google Scholar] [CrossRef]
- Stadler, K.; Li, X.; Liu, B.; Bao, W.; Wang, K.; Lehmler, H.J. Systematic review of human biomonitoring studies of ethylenethiourea, a urinary biomarker for exposure to dithiocarbamate fungicides. Environ. Pollut. 2022, 292, 118419. [Google Scholar] [CrossRef]
- Simonetti, G.; Castellani, F.; Di Filippo, P.; Riccardi, C.; Pomata, D.; Risoluti, R.; Buiarelli, F.; Sonego, E. Determination of mancozeb, a pesticide used worldwide in agriculture: Comparison among GC, LC, and CE. Curr. Anal. Chem. 2020, 16, 1041–1053. [Google Scholar] [CrossRef]
- Pirisi, F.M.; Angioni, A.; Cabizza, M.; Cabras, P.; Cao, C.F. Photolysis of pesticides: Influence of epicuticular waxes from Persica laevis DC on the photodegradation in the solid phase of aminocarb, methiocarb and fenthion. Pest. Manag. Sci. 2001, 57, 522–526. [Google Scholar] [CrossRef]
- Pirisi, F.M.; Angioni, A.; Cabizza, M.; Cabras, P.; Maccioni, E. Influence of epicuticular waxes on the photolysis of pirimicarb in the solid phase. J. Agric. Food Chem. 1998, 46, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Angioni, A.; Cabizza, M.; Cabras, M.; Melis, M.; Tuberoso, C.; Cabras, P. Effect of the epicuticular waxes of fruits and vegetables on the photodegradation of rotenone. J. Agric. Food Chem. 2004, 52, 3451–3455. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.C.; Chu, L.; Bouma, C.; Beukelman, L.; McLouth, R.; Larson, E.; Nienow, A.M. Comparison of the photodegradation of imazethapyr in aqueous solution, on epicuticular waxes, and on intact corn (Zea mays) and soybean (Glycine max) leaves. J. Environ. Sci. Health B 2019, 54, 129–137. [Google Scholar] [CrossRef]
- Trivella, A.S.; Monadjemi, S.; Worrall, D.R.; Kirkpatrick, I.; Arzoumanian, E.; Richard, C. Perinaphthenone phototransformation in a model of leaf epicuticular waxes. J. Photochem. 2014, 130, 93–101. [Google Scholar] [CrossRef]
- Riccio, R.; Trevisan, M.; Capri, E. Effect of surface waxes on the persistence of chlorpyrifos-methyl in apples, strawberries and grapefruits. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2006, 23, 683–692. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández, O.; Rial-Otero, R.; Simal-Gándara, J. Factors governing the removal of mancozeb residues from lettuces with washing solutions. Food Control 2013, 34, 530–538. [Google Scholar] [CrossRef]
- Hunsche, M.; Bringe, K.; Schmitz-Eiberger, M.; Noga, G. Leaf surface characteristics of apple seedlings, bean seedlings and kohlrabi plants and their impact on the retention and rainfastness of mancozeb. Pest. Manag. Sci. 2006, 62, 839–847. [Google Scholar] [CrossRef]
- Wang, Z.L.; Pang, J.X.; Liao, C.X.; Zhang, Q.H.; Sun, D.L. Determination of etoxazole in different parts of citrus fruit and its potential dietary exposure risk assessment. Chemosphere 2021, 268, 128832. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Chen, X.; Liu, F.; Pan, C. Residue distribution, dissipation behavior, and removal of four fungicide residues on harvested apple after waxing treatment. J. Agric. Food Chem. 2019, 67, 2307–2312. [Google Scholar] [CrossRef]
- Calvaruso, E.; Cammilleri, G.; Pulvirenti, A.; Lo Dico, G.M.; Lo Cascio, G.; Giaccone, V.; Vitale Badaco, V.; Ciprì, V.; Alessandra, M.M.; Vella, A.; et al. Residues of 165 pesticides in citrus fruits using LC-MS/MS: A study of the pesticides distribution from the peel to the pulp. Nat. Prod. Rep. 2020, 34, 34–38. [Google Scholar] [CrossRef]
- Tao, X.; Huang, Y.; Wang, C.; Chen, F.; Yang, L.; Ling, L.; Che, Z.; Chen, X. Recent developments in molecular docking technology applied in food science: A review. Int. J. Food Sci. Tech. 2019, 55, 33–45. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Prog. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Stammitti, L.; Derridj, S.; Garrec, J.P. Leaf epicuticular lipids of Prunus laurocerasus: Importance of extraction methods. Phytochemistry 1996, 43, 45–48. [Google Scholar] [CrossRef]
- Chen, S.-J.; Li, Y.-C.; Bi, Y.; Yin, Y.; Ge, Y.-H.; Wang, Y. Solvent effects on the ultrastructure and chemical composition of cuticular wax and its potential bioactive role against alternaria alternata in pingguoli pear. J. Integr. Agr. 2014, 13, 1137–1145. [Google Scholar] [CrossRef]
- Koch, K.; Ensikat, H.J. The hydrophobic coatings of plant surfaces: Epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 2008, 39, 759–772. [Google Scholar] [CrossRef]
- Pensec, F.; Paczkowski, C.; Grabarczyk, M.; Wozniak, A.; Benard-Gellon, M.; Bertsch, C.; Chong, J.; Szakiel, A. Changes in the triterpenoid content of cuticular waxes during fruit ripening of eight grape (Vitis vinifera) cultivars grown in the Upper Rhine Valley. J. Agric. Food Chem. 2014, 62, 7998–8007. [Google Scholar] [CrossRef]
- Radler, F. The wax coating on wine berries and its composition. Wein Wiss. 1968, 23, 295–308. [Google Scholar]
- Yang, M.; Luo, Z.; Gao, S.; Belwal, T.; Wang, L.; Qi, M.; Ban, Z.; Wu, B.; Wang, F.; Li, L. The chemical composition and potential role of epicuticular and intracuticular wax in four cultivars of table grapes. Postharvest Biol. Technol. 2021, 173, 111430. [Google Scholar] [CrossRef]
- Brizzolara, S.; Minnocci, A.; Yembaturova, E.; Tonutti, P. Ultrastructural analysis of berry skin from four grapes varieties at harvest and in relation to postharvest dehydration. Oeno One 2020, 54, 1021–1031. [Google Scholar] [CrossRef]
- Shin, K.H.; Park, H.S. Change of epicuticular waxes by formulation of fungicides in ‘Campbell Early’ grapes. Korean J. Hortic. 2010, 28, 762–767. [Google Scholar]
- Lichston, J.E.; de Godoy, S.A.P. Morphology and epicuticular wax content of coffee leaves after fungicide application. Pesqui. Agropecu. Bras. 2006, 41, 919–926. [Google Scholar] [CrossRef]
- Giacinti, G.; Raynaud, C.; Capblancq, S.; Simon, V. Evaluation and prevention of the negative matrix effect of terpenoids on pesticides in apples quantification by gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2017, 1483, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Giacinti, G.; Raynaud, C.; Capblancq, S.; Simon, V. Matrix-matching as an improvement strategy for the detection of pesticide residues. J. Food Sci. 2016, 81, T1342–T1350. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, Q. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Van der Waals potential: An important complement to molecular electrostatic potential in studying intermolecular interactions. J. Mol. Model. 2020, 26, 315. [Google Scholar] [CrossRef]
- Chen, T.; Tang, Z.; Hu, C. The combination of terahertz spectroscopy and density functional theory for vibrational modes and weak interactions analysis of vanillin derivatives. J. Mol. Struct. 2022, 1265, 133404. [Google Scholar] [CrossRef]
- Cappiello, A.; Famiglini, G.; Palma, P.; Trufelli, H. Matrix effects in liquid chromatography-mass spectrometry. J. Liq. Chromatogr. R. T 2010, 33, 1067–1081. [Google Scholar] [CrossRef]
- Casado, C.G.; Heredia, A. Ultrastructure of the cuticle during growth of the grape berry (Vitis vinifera L.). Physiol. Plant. 2001, 111, 220–224. [Google Scholar] [CrossRef]
- Mao, H.; Lv, Y.; Chen, G.; Jiang, Y. Effects of cuticular wax on the postharvest physiology in fragrant pear at different storages. J. Sci. Food Agr. 2022, 102, 4425–4434. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández, O.; Rial-Otero, R.; González-Barreiro, C.; Simal-Gándara, J. Surveillance of fungicidal dithiocarbamate residues in fruits and vegetables. Food Chem. 2012, 134, 366–374. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, B.; Wen, A.; Yu, H.; Guo, Y.; Cheng, Y.; Xie, Y.; Qian, H.; Yao, W. Interaction between Six Waxy Components in Summer Black Grapes (Vitis vinifera) and Mancozeb and Its Effect on the Residue of Mancozeb. Int. J. Mol. Sci. 2023, 24, 7705. https://doi.org/10.3390/ijms24097705
Guo B, Wen A, Yu H, Guo Y, Cheng Y, Xie Y, Qian H, Yao W. Interaction between Six Waxy Components in Summer Black Grapes (Vitis vinifera) and Mancozeb and Its Effect on the Residue of Mancozeb. International Journal of Molecular Sciences. 2023; 24(9):7705. https://doi.org/10.3390/ijms24097705
Chicago/Turabian StyleGuo, Boru, Aying Wen, Hang Yu, Yahui Guo, Yuliang Cheng, Yunfei Xie, He Qian, and Weirong Yao. 2023. "Interaction between Six Waxy Components in Summer Black Grapes (Vitis vinifera) and Mancozeb and Its Effect on the Residue of Mancozeb" International Journal of Molecular Sciences 24, no. 9: 7705. https://doi.org/10.3390/ijms24097705