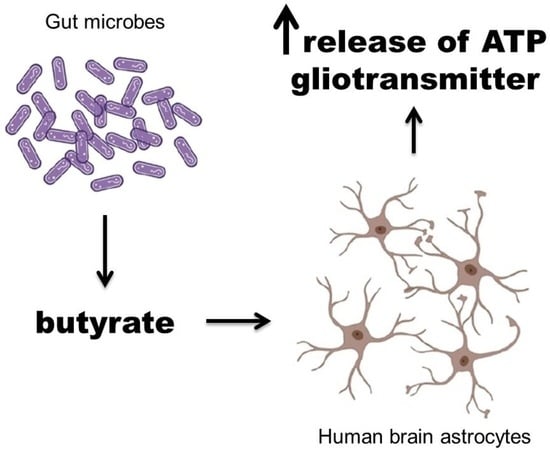
Gut Microbiota Metabolites Differentially Release Gliotransmitters from the Cultured Human Astrocytes: A Preliminary Report
Abstract
:1. Introduction
2. Results
2.1. The Effect of the Tested Gut Metabolites on Cell Viability
2.2. ATP and Glu Release Dynamics
2.3. ATP and Glu Release in Response to Control Compounds
2.4. The Effects of the Tested Gut Metabolites on ATP and Glu Release
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Human Astrocyte Cell Culture
4.3. Cell Viability MTT Test
4.4. ATP and Glu Release Dynamics
4.5. Effect of the Control and Tested Compounds on ATP and Glu Release
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, S.F.; Tauber, A.I. Rethinking Individuality: The Dialectics of the Holobiont. Biol. Philos. 2016, 31, 839–853. [Google Scholar] [CrossRef]
- Dash, S.; Syed, Y.A.; Khan, M.R. Understanding the Role of the Gut Microbiome in Brain Development and Its Association With Neurodevelopmental Psychiatric Disorders. Front. Cell Dev. Biol. 2022, 10, 880544. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Frankiensztajn, L.M.; Elliott, E.; Koren, O. The Microbiota and the Hypothalamus-Pituitary-Adrenocortical (HPA) Axis, Implications for Anxiety and Stress Disorders. Curr. Opin. Neurobiol. 2020, 62, 76–82. [Google Scholar] [CrossRef]
- Tillmann, S.; Awwad, H.M.; Eskelund, A.R.; Treccani, G.; Geisel, J.; Wegener, G.; Obeid, R. Probiotics Affect One-Carbon Metabolites and Catecholamines in a Genetic Rat Model of Depression. Mol. Nutr. Food Res. 2018, 62, e1701070. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Dehghani, F.; Abdollahi, S.; Shidfar, F.; Clark, C.C.T.; Soltani, S. Probiotics Supplementation and Brain-Derived Neurotrophic Factor (BDNF): A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Neurosci. 2022, 1–11. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut Microbial Metabolites as Multi-Kingdom Intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Descamps, H.C.; Herrmann, B.; Wiredu, D.; Thaiss, C.A. The Path toward Using Microbial Metabolites as Therapies. EBioMedicine 2019, 44, 747–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short Chain Fatty Acids: Microbial Metabolites for Gut-Brain Axis Signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valvassori, S.S.; Varela, R.B.; Arent, C.O.; Dal-Pont, G.C.; Bobsin, T.S.; Budni, J.; Reus, G.Z.; Quevedo, J. Sodium Butyrate Functions as an Antidepressant and Improves Cognition with Enhanced Neurotrophic Expression in Models of Maternal Deprivation and Chronic Mild Stress. Curr. Neurovasc. Res. 2014, 11, 359–366. [Google Scholar] [CrossRef]
- Valvassori, S.S.; Resende, W.R.; Budni, J.; Dal-Pont, G.C.; Bavaresco, D.V.; Reus, G.Z.; Carvalho, A.F.; Goncalves, C.L.; Furlanetto, C.B.; Streck, E.L.; et al. Sodium Butyrate, a Histone Deacetylase Inhibitor, Reverses Behavioral and Mitochondrial Alterations in Animal Models of Depression Induced by Early- or Late-Life Stress. Curr. Neurovasc. Res. 2015, 12, 312–320. [Google Scholar] [CrossRef]
- Resende, W.R.; Valvassori, S.S.; Réus, G.Z.; Varela, R.B.; Arent, C.O.; Ribeiro, K.F.; Bavaresco, D.V.; Andersen, M.L.; Zugno, A.I.; Quevedo, J. Effects of Sodium Butyrate in Animal Models of Mania and Depression: Implications as a New Mood Stabilizer. Behav. Pharmacol. 2013, 24, 569. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.B.; Melas, P.A.; Wegener, G.; Mathé, A.A.; Lavebratt, C. Antidepressant-Like Effect of Sodium Butyrate Is Associated with an Increase in TET1 and in 5-Hydroxymethylation Levels in the Bdnf Gene. Int. J. Neuropsychopharmacol. 2015, 18, pyu032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Huang, Q.; Xu, H.; Niu, L.; Zhou, J.-N. Antidepressant-like Effects of Sodium Butyrate in Combination with Estrogen in Rat Forced Swimming Test: Involvement of 5-HT1A Receptors. Behav. Brain Res. 2009, 196, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, Y.; Fuchikami, M.; Morinobu, S.; Segawa, M.; Matsumoto, T.; Yamawaki, S. Antidepressant-like Effect of Sodium Butyrate (HDAC Inhibitor) and Its Molecular Mechanism of Action in the Rat Hippocampus. World J. Biol. Psychiatry 2012, 13, 458–467. [Google Scholar] [CrossRef]
- Yamawaki, Y.; Yoshioka, N.; Nozaki, K.; Ito, H.; Oda, K.; Harada, K.; Shirawachi, S.; Asano, S.; Aizawa, H.; Yamawaki, S.; et al. Sodium Butyrate Abolishes Lipopolysaccharide-Induced Depression-like Behaviors and Hippocampal Microglial Activation in Mice. Mol. Brain Res. 2018, 1680, 13–38. [Google Scholar] [CrossRef]
- Schroeder, F.A.; Lin, C.L.; Crusio, W.E.; Akbarian, S. Antidepressant-Like Effects of the Histone Deacetylase Inhibitor, Sodium Butyrate, in the Mouse. Biol. Psychiatry 2007, 1, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Garcez, M.L.; Tan, V.X.; Heng, B.; Guillemin, G.J. Sodium Butyrate and Indole-3-Propionic Acid Prevent the Increase of Cytokines and Kynurenine Levels in LPS-Induced Human Primary Astrocytes. Int. J. Tryptophan Res. 2020, 13, 1178646920978404. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics Analysis Reveals Large Effects of Gut Microflora on Mammalian Blood Metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [Green Version]
- Konopelski, P.; Mogilnicka, I. Biological Effects of Indole-3-Propionic Acid, a Gut Microbiota-Derived Metabolite, and Its Precursor Tryptophan in Mammals’ Health and Disease. Int. J. Mol. Sci. 2022, 23, 1222. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Harada, K.; Kamiya, T.; Tsuboi, T. Gliotransmitter Release from Astrocytes: Functional, Developmental and Pathological Implications in the Brain. Front. Neurosci. 2016, 9, 499. [Google Scholar] [CrossRef] [Green Version]
- Pillai, A.G.; Nadkarni, S. Amyloid Pathology Disrupts Gliotransmitter Release in Astrocytes. PLOS Comput. Biol. 2022, 18, e1010334. [Google Scholar] [CrossRef]
- Piacentini, R.; Puma, D.D.L.; Mainardi, M.; Lazzarino, G.; Tavazzi, B.; Arancio, O.; Grassi, C. Reduced Gliotransmitter Release from Astrocytes Mediates Tau-Induced Synaptic Dysfunction in Cultured Hippocampal Neurons. Glia 2017, 65, 1302–1316. [Google Scholar] [CrossRef]
- Boué-Grabot, E.; Pankratov, Y. Modulation of Central Synapses by Astrocyte-Released ATP and Postsynaptic P2X Receptors. Neural Plast. 2017, 2017, 9454275. [Google Scholar] [CrossRef] [Green Version]
- Illes, P.; Rubini, P.; Yin, H.; Tang, Y. Impaired ATP Release from Brain Astrocytes May Be a Cause of Major Depression. Neurosci. Bull. 2020, 36, 1281–1284. [Google Scholar] [CrossRef]
- Lin, S.; Huang, L.; Luo, Z.; Li, X.; Jin, S.; Du, Z.; Wu, D.; Xiong, W.; Huang, L.; Luo, Z.; et al. The ATP Level in the Medial Prefrontal Cortex Regulates Depressive-like Behavior via the Medial Prefrontal Cortex-Lateral Habenula Pathway. Biol. Psychiatry 2022, 92, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. Amyotrophic Lateral Sclerosis Pathoetiology and Pathophysiology: Roles of Astrocytes, Gut Microbiome, and Muscle Interactions via the Mitochondrial Melatonergic Pathway, with Disruption by Glyphosate-Based Herbicides. Int. J. Mol. Sci. 2023, 24, 587. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, L.-P.; Wang, Q.; Wu, Q.; Hu, H.-H.; Zhang, M.; Fang, Y.-Y.; Zhang, J.; Li, S.-J.; Xiong, W.-C.; et al. Astrocyte-Derived ATP Modulates Depressive-like Behaviors. Nat. Med. 2013, 19, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Spichak, S.; Donoso, F.; Moloney, G.M.; Gunnigle, E.; Brown, J.M.; Codagnone, M.; Dinan, T.G.; Cryan, J.F. Microbially-Derived Short-Chain Fatty Acids Impact Astrocyte Gene Expression in a Sex-Specific Manner. Brain Behav. Immun. Health 2021, 16, 100318. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, D.; Weng, F.; Jin, Y.; He, L. Sodium Butyrate Ameliorates the Cognitive Impairment of Alzheimer’s Disease by Regulating the Metabolism of Astrocytes. Psychopharmacology 2022, 239, 215–227. [Google Scholar] [CrossRef]
- Yang, T.; Rodriguez, V.; Malphurs, W.L.; Schmidt, J.T.; Ahmari, N.; Sumners, C.; Martyniuk, C.J.; Zubcevic, J. Butyrate Regulates Inflammatory Cytokine Expression without Affecting Oxidative Respiration in Primary Astrocytes from Spontaneously Hypertensive Rats. Physiol. Rep. 2018, 6, e13732. [Google Scholar] [CrossRef] [Green Version]
- Falomir-Lockhart, L.J.; Cavazzutti, G.F.; Giménez, E.; Toscani, A.M. Fatty Acid Signaling Mechanisms in Neural Cells: Fatty Acid Receptors. Front. Cell. Neurosci. 2019, 13, 162. [Google Scholar] [CrossRef] [Green Version]
- Rothhammer, V.; Kenison, J.E.; Li, Z.; Tjon, E.; Takenaka, M.C.; Chao, C.-C.; de Lima, K.A.; Borucki, D.M.; Kaye, J.; Quintana, F.J. Aryl Hydrocarbon Receptor Activation in Astrocytes by Laquinimod Ameliorates Autoimmune Inflammation in the CNS. Neurol. Neuroimmunol. Neuroinflammat. 2021, 8, e946. [Google Scholar] [CrossRef]
- Miletta, M.C.; Petkovic, V.; Eblé, A.; Ammann, R.A.; Flück, C.E.; Mullis, P.-E. Butyrate Increases Intracellular Calcium Levels and Enhances Growth Hormone Release from Rat Anterior Pituitary Cells via the G-Protein-Coupled Receptors GPR41 and 43. PLoS ONE 2014, 9, e107388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR Signaling Pathways and Regulatory Functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Sun, S.; Teng, S.; Jin, M.; Zhou, Z. Ca2+-Dependent and Ca2+-Independent ATP Release in Astrocytes. Front. Mol. Neurosci. 2018, 11, 224. [Google Scholar] [CrossRef]
- Kuga, N.; Sasaki, T.; Takahara, Y.; Matsuki, N.; Ikegaya, Y. Large-Scale Calcium Waves Traveling through Astrocytic Networks In Vivo. J. Neurosci. 2011, 31, 2607–2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grković, I.; Drakulić, D.; Martinović, J.; Mitrović, N. Role of Ectonucleotidases in Synapse Formation During Brain Development: Physiological and Pathological Implications. Curr. Neuropharmacol. 2019, 17, 84–98. [Google Scholar] [CrossRef]
- Dedkova, E.N.; Sigova, A.A.; Zinchenko, V.P. Mechanism of Action of Calcium Ionophores on Intact Cells: Ionophore-Resistant Cells. Membr. Cell Biol. 2000, 13, 357–368. [Google Scholar]
- Kim, H.; Lee, B.-H.; Choi, S.-H.; Kim, H.-J.; Jung, S.-W.; Hwang, S.-H.; Rhim, H.; Kim, H.-C.; Cho, I.-H.; Nah, S.-Y. Gintonin Stimulates Gliotransmitter Release in Cortical Primary Astrocytes. Neurosci. Lett. 2015, 603, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kárpáti, A.; Yoshikawa, T.; Nakamura, T.; Iida, T.; Matsuzawa, T.; Kitano, H.; Harada, R.; Yanai, K. Histamine Elicits Glutamate Release from Cultured Astrocytes. J. Pharmacol. Sci. 2018, 137, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Olsson, L.M.; Boulund, F.; Nilsson, S.; Khan, M.T.; Gummesson, A.; Fagerberg, L.; Engstrand, L.; Perkins, R.; Uhlén, M.; Bergström, G.; et al. Dynamics of the Normal Gut Microbiota: A Longitudinal One-Year Population Study in Sweden. Cell Host Microbe 2022, 30, 726–739.e3. [Google Scholar] [CrossRef]
- Kinoshita, M.; Hirayama, Y.; Fujishita, K.; Shibata, K.; Shinozaki, Y.; Shigetomi, E.; Takeda, A.; Le, H.P.N.; Hayashi, H.; Hiasa, M.; et al. Anti-Depressant Fluoxetine Reveals Its Therapeutic Effect Via Astrocytes. EBioMedicine 2018, 32, 72–83. [Google Scholar] [CrossRef]
- Jun, M.; Xiaolong, Q.; Chaojuan, Y.; Ruiyuan, P.; Shukun, W.; Junbing, W.; Li, H.; Hong, C.; Jinbo, C.; Rong, W.; et al. Calhm2 Governs Astrocytic ATP Releasing in the Development of Depression-like Behaviors. Mol. Psychiatry 2018, 23, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, S. Glial Purinergic Signals and Psychiatric Disorders. Front. Cell. Neurosci. 2022, 15, 822614. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, K. Effects of Butyrate on Affective Processes. Clinical Trial Registration NCT04722549; clinicaltrials.gov; 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04722549 (accessed on 11 July 2021).
- Chatterjee, S. Microbiome Targeted Oral Butyrate Therapy in Gulf War Multisymptom Illness. Clinical Trial Registration NCT05367245; clinicaltrials.gov; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05367245 (accessed on 13 February 2023).
- Bachmann, C.; Colombo, J.-P.; Berüter, J. Short Chain Fatty Acids in Plasma and Brain: Quantitative Determination by Gas Chromatography. Clin. Chim. Acta 1979, 92, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Cayman Chemical. Sodium Butyrate. Product Information. 2014. Available online: https://fnkprddata.blob.core.windows.net/domestic/data/datasheet/CAY/13121.pdf (accessed on 19 February 2023).
- Liu, J.; Sun, J.; Wang, F.; Yu, X.; Ling, Z.; Li, H.; Zhang, H.; Jin, J.; Chen, W.; Pang, M.; et al. Neuroprotective Effects of Clostridium Butyricum against Vascular Dementia in Mice via Metabolic Butyrate. BioMed Res. Int. 2015, 2015, 412946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, T.W.; Brockway, J.A.; Higgins, L.S. The Density of Tissues in and About the Head. Acta Neurol. Scand. 1970, 46, 85–92. [Google Scholar] [CrossRef]
- Yang, T.; Magee, K.L.; Colon-Perez, L.M.; Larkin, R.; Liao, Y.-S.; Balazic, E.; Cowart, J.R.; Arocha, R.; Redler, T.; Febo, M.; et al. Impaired Butyrate Absorption in the Proximal Colon, Low Serum Butyrate and Diminished Central Effects of Butyrate on Blood Pressure in Spontaneously Hypertensive Rats. Acta Physiol. Oxf. Engl. 2019, 226, e13256. [Google Scholar] [CrossRef]
- Smith, R.C. Sodium Butyrate as a Treatment for Improving Cognitive Function in Schizophrenia. Clinical Trial Registration NCT02654405; clinicaltrials.gov; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02654405 (accessed on 13 February 2023).
- Dalile, B.; Vervliet, B.; Bergonzelli, G.; Verbeke, K.; Van Oudenhove, L. Colon-Delivered Short-Chain Fatty Acids Attenuate the Cortisol Response to Psychosocial Stress in Healthy Men: A Randomized, Placebo-Controlled Trial. Neuropsychopharmacology 2020, 45, 2257–2266. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Hung, C.-C.; Hsu, P.-C.; Lee, P.-Y.; Tsai, Y.-A.; Hsin, Y.-C.; Lee, X.-T.; Chou, C.-C.; Chen, M.-L.; Tarng, D.-C.; et al. Astrocytic Aryl Hydrocarbon Receptor Mediates Chronic Kidney Disease-Associated Mental Disorders Involving GLT1 Hypofunction and Neuronal Activity Enhancement in the Mouse Brain. Glia 2022, 9, 499. [Google Scholar] [CrossRef]
- Parpura, V.; Basarsky, T.A.; Liu, F.; Jeftinija, K.; Jeftinija, S.; Haydon, P.G. Glutamate-Mediated Astrocyte-Neuron Signalling. Nature 1994, 369, 744–747. [Google Scholar] [CrossRef]
- Ohmomo, H.; Ehara, A.; Yoshida, S.; Shutoh, F.; Ueda, S.; Hisano, S. Temporally Distinct Expression of Vesicular Glutamate Transporters 1 and 2 during Embryonic Development of the Rat Olfactory System. Neurosci. Res. 2011, 70, 376–382. [Google Scholar] [CrossRef]
- Lupton, J.R. Microbial Degradation Products Influence Colon Cancer Risk: The Butyrate Controversy. J. Nutr. 2004, 134, 479–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karbownik, M.S.; Sokołowska, P.; Kowalczyk, E. Gut Microbiota Metabolites Differentially Release Gliotransmitters from the Cultured Human Astrocytes: A Preliminary Report. Int. J. Mol. Sci. 2023, 24, 6617. https://doi.org/10.3390/ijms24076617
Karbownik MS, Sokołowska P, Kowalczyk E. Gut Microbiota Metabolites Differentially Release Gliotransmitters from the Cultured Human Astrocytes: A Preliminary Report. International Journal of Molecular Sciences. 2023; 24(7):6617. https://doi.org/10.3390/ijms24076617
Chicago/Turabian StyleKarbownik, Michał Seweryn, Paulina Sokołowska, and Edward Kowalczyk. 2023. "Gut Microbiota Metabolites Differentially Release Gliotransmitters from the Cultured Human Astrocytes: A Preliminary Report" International Journal of Molecular Sciences 24, no. 7: 6617. https://doi.org/10.3390/ijms24076617