Neuronal Prosurvival Role of Ceramide Synthase 2 by Olidogendrocyte-to-Neuron Extracellular Vesicle Transfer
Abstract
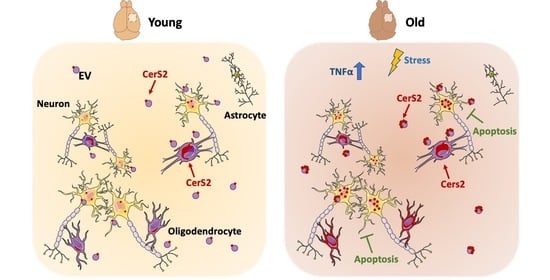
:1. Introduction
2. Results
2.1. Age Leads to a Reduced Number of Cortical Extracellular Vesicles, Which Are Enriched in the Oligodendrocyte Enzyme CerS2
2.2. CerS2 Is Present in Oligodendrocytes and in Cortical Neurons In Vivo
2.3. Neuronal CerS2 Originates from Oligodendrocyte-Derived EVs
2.4. Inflammatory Stimuli Lead to Increased CerS2, Which Contributes to Neuronal Survival When Present in EVs
3. Discussion
4. Materials and Methods
4.1. Experimentation Animals
4.2. Primary Cortical Neurons Cultures
4.3. Primary Oligodendrocyte Culture
4.4. SH-SY5Y Cell Line
4.5. HOG Cell Line
4.6. Generation of HOG-Veh and HOG-CerS2 Lines
4.7. HEK Cell Line
4.8. Isolation of EVs from Mouse Cerebral Cortex Tissue
4.9. Isolation of EVs from Cell Culture Medium
4.10. Labelling Exosomes with Bodipy
4.11. Protein Identification by Western Blot
4.12. Immunofluorescence Labelling
4.13. Transmission Electron Microscopy
4.14. Proteomics
4.15. Artificial Intelligence Bioinformatics Analysis
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charlton, R.A.; Schiavone, F.; Barrick, T.R.; Morris, R.G.; Markus, H.S. Diffusion tensor imaging detects age related white matter change over a 2 year follow-up which is associated with working memory decline. J. Neurol. Neurosurg. Psychiatry 2010, 81, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlton, R.A.; Barrick, T.R.; McIntyre, D.J.; Shen, Y.; O’Sullivan, M.; Howe, F.A.; Clark, C.A.; Morris, R.G.; Markus, H.S. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology 2006, 66, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Coffey, C.E.; Ratcliff, G.; Saxton, J.A.; Bryan, R.N.; Fried, L.P.; Lucke, J.F. Cognitive Correlates of Human Brain Aging. J. Neuropsychiatry Clin. Neurosci. 2001, 13, 471–485. [Google Scholar] [CrossRef]
- Morris, J.; Mcmanus, D.Q. The neurology of aging: Normal versus pathologic change. Geriatrics 1991, 46, 47–54. [Google Scholar]
- Salthouse, T.A. Working-memory mediationof adult age differences in integrativereasoning. Mem. Cognit 1992, 20, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiavone, F.; Charlton, R.A.; Barrick, T.R.; Morris, R.G.; Markus, H.S. Imaging age-related cognitive decline: A comparison of diffusion tensor and magnetization transfer MRI. J. Magn. Reson. Imaging 2009, 29, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Vernooij, M.W.; de Groot, M.; van der Lugt, A.; Ikram, M.A.; Krestin, G.P.; Hofman, A.; Niessen, W.J.; Breteler, M.M. White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. Neuroimage 2008, 43, 470–477. [Google Scholar] [CrossRef]
- Burke, S.N.; Barnes, C.A. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci. 2010, 33, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Morrison, J.H.; Hof, P.R. Life and Death of Neurons in the Aging Brain. Science (80-) 1997, 278, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Nyengaard, J.; Pakkenberg, B.; Gundersen, H.J. Age-Induced White Matter Changes in the Human Brain: A Stereological Investigation. Neurobiol. Aging 1997, 18, 609–615. [Google Scholar] [CrossRef]
- Yankner, B.A.; Lu, T.; Loerch, P. The Aging Brain. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Sandell, J.H.; Peters, A. Effects of age on nerve fibers in the rhesus monkey optic nerve. J. Comp. Neurol. 2001, 429, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Sandell, J.H.; Peters, A. Effects of age on the glial cells in the rhesus monkey optic nerve. J. Comp. Neurol. 2002, 445, 13–28. [Google Scholar] [CrossRef]
- Peters, A.; Sethares, C. Oligodendrocytes, their Progenitors and other Neuroglial Cells in the Aging Primate Cerebral Cortex. Cereb. Cortex 2004, 14, 995–1007. [Google Scholar] [CrossRef]
- Levison, S.W.; Young, G.M.; Goldman, J.E. Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J. Neurosci. Res. 1999, 57, 435–446. [Google Scholar] [CrossRef]
- Lasiene, J.; Matsui, A.; Sawa, Y.; Wong, F.; Horner, P.J. Age-related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging Cell 2009, 8, 201–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Schnatz, A.; Müller, C.; Brahmer, A.; Krämer-Albers, E. Extracellular Vesicles in neural cell interaction and CNS homeostasis. FASEB BioAdvances 2021, 3, 577–592. [Google Scholar] [CrossRef]
- Nutma, E.; van Gent, D.; Amor, S.; Peferoen, L.A.N. Astrocyte and Oligodendrocyte Cross-Talk in the Central Nervous System. Cells 2020, 9, 600. [Google Scholar] [CrossRef] [Green Version]
- Pascual, M.; Ibáñez, F.; Guerri, C. Exosomes as mediators of neuron-glia communication in neuroinflammation. Neural Regen. Res. 2020, 15, 796. [Google Scholar] [CrossRef]
- Frühbeis, C.; Kuo-Elsner, W.P.; Müller, C.; Barth, K.; Peris, L.; Tenzer, S.; Möbius, W.; Werner, H.B.; Nave, K.A.; Fröhlich, D.; et al. Oligodendrocytes support axonal transport and maintenance via exosome secretion. PLoS Biol. 2020, 18, e3000621. [Google Scholar] [CrossRef] [PubMed]
- Tietje, A.; Maron, K.N.; Wei, Y.; Feliciano, D.M. Cerebrospinal Fluid Extracellular Vesicles Undergo Age Dependent Declines and Contain Known and Novel Non-coding RNAs. PLoS ONE 2014, 9, e113116. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.; Sun, H.; Usuki, S.; Sakai, S.; Hanamatsu, H.; Mioka, T.; Kimura, N.; Okada, M.; Tahara, H.; Furukawa, J.; et al. A potential function for neuronal exosomes: Sequestering intracerebral amyloid-β peptide. FEBS Lett. 2015, 589, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Kapogiannis, D.; Schwartz, J.B.; Lobach, I.V.; Goetzl, L.; Abner, E.L.; Jicha, G.A.; Karydas, A.M.; Boxer, A.; Miller, B.L. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J. 2016, 30, 4141–4148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Anca, M.; Fenoglio, C.; Serpente, M.; Arosio, B.; Cesari, M.; Scarpini, E.A.; Galimberti, D. Exosome Determinants of Physiological Aging and Age-Related Neurodegenerative Diseases. Front. Aging Neurosci. 2019, 11, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; Feng, X.; Wang, H.; Xu, W.; Zhao, Y.; Ma, W.; Jiang, S.; Liu, D.; Huang, J.; Songyang, Z. Mir-23a induces telomere dysfunction and cellular senescence by inhibiting TRF 2 expression. Aging Cell 2015, 14, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Terlecki-Zaniewicz, L.; Lämmermann, I.; Latreille, J.; Bobbili, M.R.; Pils, V.; Schosserer, M.; Weinmüllner, R.; Dellago, H.; Skalicky, S.; Pum, D.; et al. Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype. Aging 2018, 10, 1103–1132. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef] [Green Version]
- Imgrund, S.; Hartmann, D.; Farwanah, H.; Eckhardt, M.; Sandhoff, R.; Degen, J.; Gieselmann, V.; Sandhoff, K.; Willecke, K. Adult Ceramide Synthase 2 (CERS2)-deficient Mice Exhibit Myelin Sheath Defects, Cerebellar Degeneration, and Hepatocarcinomas. J. Biol. Chem. 2009, 284, 33549–33560. [Google Scholar] [CrossRef] [Green Version]
- Coetzee, T.; Fujita, N.; Dupree, J.; Shi, R.; Blight, A.; Suzuki, K.; Suzuki, K.; Popko, B. Myelination in the Absence of Galactocerebroside and Sulfatide: Normal Structure with Abnormal Function and Regional Instability. Cell 1996, 86, 209–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosio, A.; Binczek, E.; Stoffel, W. Functional breakdown of the lipid bilayer of the myelin membrane in central and peripheral nervous system by disrupted galactocerebroside synthesis. Proc. Natl. Acad. Sci. USA 1996, 93, 13280–13285. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, D.; Wegner, M.S.; Wanger, R.A.; Ferreirós, N.; Schreiber, Y.; Lucks, J.; Schiffmann, S.; Geisslinger, G.; Grösch, S. The equilibrium between long and very long chain ceramides is important for the fate of the cell and can be influenced by co-expression of CerS. Int. J. Biochem. Cell Biol. 2013, 45, 1195–1203. [Google Scholar] [CrossRef]
- Mesicek, J.; Lee, H.; Feldman, T.; Jiang, X.; Skobeleva, A.; Berdyshev, E.V.; Haimovitz-Friedman, A.; Fuks, Z.; Kolesnick, R. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal. 2010, 22, 1300–1307. [Google Scholar] [CrossRef] [Green Version]
- Sassa, T.; Suto, S.; Okayasu, Y.; Kihara, A. A shift in sphingolipid composition from C24 to C16 increases susceptibility to apoptosis in HeLa cells. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids. 2012, 1821, 1031–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, B.A.; Liao, X.; Moore, K.S.; Southard, A.; Roddy, P.; Ji, R.; Szulc, Z.; Bielawska, A.; Schulze, P.C.; Cowart, L.A. Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J. 2018, 32, 1403–1416. [Google Scholar] [CrossRef] [Green Version]
- Siskind, L.J.; Mullen, T.D.; Romero Rosales, K.; Clarke, C.J.; Hernandez-Corbacho, M.J.; Edinger, A.L.; Obeid, L.M. The BCL-2 Protein BAK Is Required for Long-chain Ceramide Generation during Apoptosis. J. Biol. Chem. 2010, 285, 11818–11826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Yu, W.; Gong, M.; You, J.; Liu, J.; Zheng, J. Overexpression of a Novel Tumor Metastasis Suppressor Gene TMSG1/LASS2 Induces Apoptosis via a Caspase-dependent Mitochondrial Pathway. J. Cell Biochem. 2015, 116, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Ximerakis, M.; Lipnick, S.L.; Innes, B.T.; Simmons, S.K.; Adiconis, X.; Dionne, D.; Mayweather, B.A.; Nguyen, L.; Niziolek, Z.; Ozek, C.; et al. Single-cell transcriptomic profiling of the aging mouse brain. Nat. Neurosci. 2019, 22, 1696–1708. [Google Scholar] [CrossRef]
- Becker, I.; Wang-Eckhardt, L.; Yaghootfam, A.; Gieselmann, V.; Eckhardt, M. Differential expression of (dihydro)ceramide synthases in mouse brain: Oligodendrocyte-specific expression of CerS2/Lass2. Histochem. Cell Biol. 2008, 129, 233–241. [Google Scholar] [CrossRef]
- Kremser, C.; Klemm, A.L.; van Uelft, M.; Imgrund, S.; Ginkel, C.; Hartmann, D.; Willecke, K. Cell-type-specific expression pattern of ceramide synthase 2 protein in mouse tissues. Histochem. Cell Biol. 2013, 140, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.W.; Zhang, Y.; Herrup, K. Non-Neuronal Cells Are Required to Mediate the Effects of Neuroinflammation: Results from a Neuron-Enriched Culture System. PLoS ONE 2016, 11, e0147134. [Google Scholar] [CrossRef] [Green Version]
- Hilgenberg, L.G.W.; Smith, M.A. Preparation of dissociated mouse cortical neuron cultures. J. Vis. Exp. 2007, 10, 562. [Google Scholar]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E.; Bielak-Zmijewska, A.; Dudkowska, M.; Krzystyniak, A.; Mosieniak, G.; Wesierska, M.; Wlodarczyk, J. Cellular Senescence in Brain Aging. Front. Aging Neurosci. 2021, 13, 646924. [Google Scholar] [CrossRef] [PubMed]
- Jara, J.H.; Singh, B.B.; Floden, A.M.; Combs, C.K. Tumor necrosis factor alpha stimulates NMDA receptor activity in mouse cortical neurons resulting in ERK-dependent death. J. Neurochem. 2007, 100, 1407–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Jung, K.; Kim, I.S.; Lee, I.S.; Ko, Y.; Shin, J.E.; Park, K.I. TNF-α induces human neural progenitor cell survival after oxygen–glucose deprivation by activating the NF-κB pathway. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Podbielska, M.; Szulc, Z.M.; Kurowska, E.; Hogan, E.L.; Bielawski, J.; Bielawska, A.; Bhat, N.R. Cytokine-induced release of ceramide-enriched exosomes as a mediator of cell death signaling in an oligodendroglioma cell line. J. Lipid Res. 2016, 57, 2028–2039. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Ye, L.; Lu, H.; Chen, H.; Zhang, Y.; Huang, Y.; Zheng, J.C. TNF-α promotes extracellular vesicle release in mouse astrocytes through glutaminase. J. Neuroinflammation 2017, 14, 87. [Google Scholar] [CrossRef] [Green Version]
- Spassieva, S.D.; Ji, X.; Liu, Y.; Gable, K.; Bielawski, J.; Dunn, T.M.; Bieberich, E.; Zhao, L. Ectopic expression of ceramide synthase 2 in neurons suppresses neurodegeneration induced by ceramide synthase 1 deficiency. Proc. Natl. Acad. Sci. USA 2016, 113, 5928–5933. [Google Scholar] [CrossRef] [Green Version]
- Safaiyan, S.; Kannaiyan, N.; Snaidero, N.; Brioschi, S.; Biber, K.; Yona, S.; Edinger, A.L.; Jung, S.; Rossner, M.J.; Simons, M. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 2016, 19, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ren, S.Y.; Chen, J.F.; Liu, K.; Li, R.X.; Li, Z.F.; Hu, B.; Niu, J.Q.; Xiao, L.; Chan, J.R.; et al. Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat. Neurosci. 2020, 23, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Baloni, P.; Arnold, M.; Buitrago, L.; Nho, K.; Moreno, H.; Huynh, K.; Brauner, B.; Louie, G.; Kueider-Paisley, A.; Suhre, K.; et al. Multi-Omic analyses characterize the ceramide/sphingomyelin pathway as a therapeutic target in Alzheimer’s disease. Commun. Biol. 2022, 5, 1074. [Google Scholar] [CrossRef] [PubMed]
- Couttas, T.A.; Kain, N.; Suchowerska, A.K.; Quek, L.E.; Turner, N.; Fath, T.; Garner, B.; Don, A.S. Loss of ceramide synthase 2 activity, necessary for myelin biosynthesis, precedes tau pathology in the cortical pathogenesis of Alzheimer’s disease. Neurobiol. Aging 2016, 43, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Czubowicz, K.; Jęśko, H.; Wencel, P.; Lukiw, W.J.; Strosznajder, R.P. The Role of Ceramide and Sphingosine-1-Phosphate in Alzheimer’s Disease and Other Neurodegenerative Disorders. Mol. Neurobiol. 2019, 56, 5436–5455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolga, A.M.; Granic, I.; Blank, T.; Knaus, H.G.; Spiess, J.; Luiten, P.G.; Eisel, U.L.; Nijholt, I.M. TNF-alpha-mediates neuroprotection against glutamate-induced excitotoxicity via NF-kappaB-dependent up-regulation of K2.2 channels. J. Neurochem. 2008, 107, 1158–1167. [Google Scholar]
- Mukhopadhyay, S.; Hoidal, J.R.; Mukherjee, T.K. Role of TNFα in pulmonary pathophysiology. Respir. Res. 2006, 7, 125. [Google Scholar] [CrossRef] [Green Version]
- Terrando, N.; Monaco, C.; Ma, D.; Foxwell, B.M.J.; Feldmann, M.; Maze, M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc. Natl. Acad. Sci. USA 2010, 107, 20518–20522. [Google Scholar] [CrossRef] [Green Version]
- Varfolomeev, E.; Vucic, D. Intracellular regulation of TNF activity in health and disease. Cytokine 2018, 101, 26–32. [Google Scholar] [CrossRef]
- Wegner, M.-S.; Schiffmann, S.; Parnham, M.J.; Geisslinger, G.; Grösch, S. The enigma of ceramide synthase regulation in mammalian cells. Prog. Lipid Res. 2016, 63, 93–119. [Google Scholar] [CrossRef]
- Benvegnù, S.; Mateo, M.I.M.I.; Palomer, E.; Jurado-Arjona, J.; Dotti, C.G.C.G. Aging Triggers Cytoplasmic Depletion and Nuclear Translocation of the E3 Ligase Mahogunin: A Function for Ubiquitin in Neuronal Survival. Mol. Cell 2017, 66, 358–372.e7. [Google Scholar] [CrossRef] [PubMed]
- Vella, L.J.; Scicluna, B.J.; Cheng, L.; Bawden, E.G.; Masters, C.L.; Ang, C.S.; Willamson, N.; McLean, C.; Barnham, K.J.; Hill, A.F. A rigorous method to enrich for exosomes from brain tissue. J. Extracell. Vesicles 2017, 6, 1348885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorba, G.; Aguirre-Plans, J.; Junet, V.; Segú-Vergés, C.; Ruiz, J.L.; Pujol, A.; Fernández-Fuentes, N.; Mas, J.M.; Oliva, B. In-silico simulated prototype-patients using TPMS technology to study a potential adverse effect of sacubitril and valsartan. PLoS ONE 2020, 15, e0228926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, C.M. Pattern Recognition and Machine Learning; Springer: New York, NY, USA, 2007. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casadomé-Perales, Á.; Naya, S.; Fernández-Martínez, E.; Mille, B.G.; Guerrero-Valero, M.; Peinado, H.; Guix, F.X.; Dotti, C.G.; Palomer, E. Neuronal Prosurvival Role of Ceramide Synthase 2 by Olidogendrocyte-to-Neuron Extracellular Vesicle Transfer. Int. J. Mol. Sci. 2023, 24, 5986. https://doi.org/10.3390/ijms24065986
Casadomé-Perales Á, Naya S, Fernández-Martínez E, Mille BG, Guerrero-Valero M, Peinado H, Guix FX, Dotti CG, Palomer E. Neuronal Prosurvival Role of Ceramide Synthase 2 by Olidogendrocyte-to-Neuron Extracellular Vesicle Transfer. International Journal of Molecular Sciences. 2023; 24(6):5986. https://doi.org/10.3390/ijms24065986
Chicago/Turabian StyleCasadomé-Perales, Álvaro, Sara Naya, Elisa Fernández-Martínez, Bea G. Mille, Marta Guerrero-Valero, Héctor Peinado, Francesc X. Guix, Carlos G. Dotti, and Ernest Palomer. 2023. "Neuronal Prosurvival Role of Ceramide Synthase 2 by Olidogendrocyte-to-Neuron Extracellular Vesicle Transfer" International Journal of Molecular Sciences 24, no. 6: 5986. https://doi.org/10.3390/ijms24065986