Amphiphilic Polyethylene-b-poly(L-lysine) Block Copolymer: Synthesis, Self-Assembly, and Responsivity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of PE-b-PLL Block Copolymer
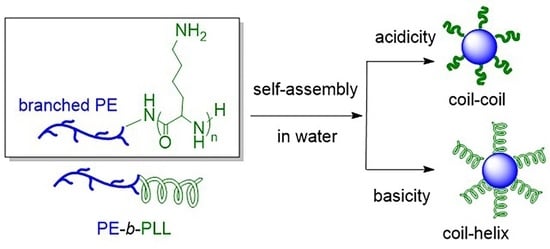
2.2. Self-Assembly of PE-b-PLL in Water
2.3. pH Responsivity of PE-b-PLL
2.4. Ionic Responsivity of PE-b-PLL
3. Materials and Methods
3.1. Synthesis of PE-b-PZL Block Copolymers
3.2. Synthesis of PE-b-PLL Block Copolymers
3.3. Preparation of the PE-b-PLL Polymeric Micelles
3.4. Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sauter, D.W.; Taoufik, M.; Boisson, C. Polyolefins, a success story. Polymers 2017, 9, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaminsky, W. Trends in Polyolefin Chemistry. Macromol. Chem. Phys. 2008, 209, 459–466. [Google Scholar] [CrossRef]
- Luo, X.; Xie, S.; Liu, J.; Hu, H.; Jiang, J.; Huang, W.; Gao, H.; Zhou, D.; Lü, Z.; Yan, D. The relationship between the degree of branching and glass transition temperature of branched polyethylene: Experiment and simulation. Polym. Chem. 2013, 5, 1305–1312. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Y.; Li, G.; Xiao, Z.; Liang, G.; Wu, Q. Catalytic synthesis of polyethylene-block-polynorbornene copolymers using a living polymerization nickel catalyst. Polym. Chem. 2014, 5, 6012–6018. [Google Scholar] [CrossRef]
- Nunvářová, K.; Charvátová, B.; Šlouf, M.; Hermanová, S.; Merna, J. Synthesis of amphiphilic copolymers based on dendritic polyethylene grafted by polyhydroxyethylmethacrylate and polyhydroxypropylmethacrylate and their use for construction of nanoparticles. Eur. Polym. J. 2019, 115, 193–200. [Google Scholar] [CrossRef]
- Olson, D.A.; Chen, L.; Hillmyer, M.A. Templating Nanoporous Polymers with Ordered Block Copolymers. Chem. Mater. 2007, 20, 869–890. [Google Scholar] [CrossRef]
- Xu, Y.; Thurber, C.M.; Lodge, T.P.; Hillmyer, M.A. Synthesis and Remarkable Efficacy of Model Polyethylene-graft-poly(methyl methacrylate) Copolymers as Compatibilizers in Polyethylene/Poly(methyl methacrylate) Blends. Macromolecules 2012, 45, 9604–9610. [Google Scholar] [CrossRef]
- Pitet, L.M.; Amendt, M.A.; Hillmyer, M.A. Nanoporous Linear Polyethylene from a Block Polymer Precursor. J. Am. Chem. Soc. 2010, 132, 8230–8231. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Subramanian, R.; Ye, Z.; Lu, J.; Yu, Q. Chain Walking Ethylene Copolymerization with an ATRP Inimer for One-Pot Synthesis of Hyperbranched Polyethylenes Tethered with ATRP Initiating Sites. Macromol. Rapid Commun. 2007, 28, 2185–2191. [Google Scholar] [CrossRef]
- Gao, H.; Tang, Y.; Hu, Z.; Guan, Q.; Shi, X.; Zhu, F.; Wu, Q. Synthesis of amphiphilic copolymers with a dendritic polyethylene core and poly(ethylene oxide) arms and their self-assembled nanostructures. Polym. Chem. 2012, 4, 1107–1114. [Google Scholar] [CrossRef]
- Song, J.; He, J.; Hu, J.; Ma, J.; Jiang, H.; Hu, S.; Ye, H.; Xu, L. A Universal Strategy for Producing Fluorescent Polymers Based on Designer Hyperbranched Polyethylene Ternary Copolymers. Macromolecules 2022, 55, 2085–2095. [Google Scholar] [CrossRef]
- Zhang, K.; Ye, Z.; Subramanian, R. Synthesis of Block Copolymers of Ethylene with Styrene and n-Butyl Acrylate via a Tandem Strategy Combining Ethylene “Living” Polymerization Catalyzed by a Functionalized Pd−Diimine Catalyst with Atom Transfer Radical Polymerization. Macromolecules 2008, 41, 640–649. [Google Scholar] [CrossRef]
- Chen, G.; Ma, X.S.; Guan, Z. Synthesis of Functional Olefin Copolymers with Controllable Topologies Using a Chain-Walking Catalyst. J. Am. Chem. Soc. 2003, 125, 6697–6704. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, X.; Gao, H.; Zhang, L.; Zhu, F.; Wu, Q. Thermo- and ph-sensitive polyethylene-based diblock and triblock copolymers: Synthesis and self-assembly in aqueous solution. J. Mater. Chem. 2012, 22, 5737–5745. [Google Scholar] [CrossRef]
- Shi, X.; Zhao, Y.; Gao, H.; Zhang, L.; Zhu, F.; Wu, Q. Synthesis of Hyperbranched Polyethylene Amphiphiles by Chain Walking Polymerization in Tandem with RAFT Polymerization and Supramolecular Self-Assembly Vesicles. Macromol. Rapid Commun. 2012, 33, 374–379. [Google Scholar] [CrossRef]
- Chen, G.; Guan, Z. Transition Metal-Catalyzed One-Pot Synthesis of Water-Soluble Dendritic Molecular Nanocarriers. J. Am. Chem. Soc. 2004, 126, 2662–2663. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, Y.C. Synthesis and Conformational Transition of Surface-Tethered Polypeptide: Poly(l-lysine). Macromolecules 2003, 36, 6511–6518. [Google Scholar] [CrossRef]
- Zheng, M.; Pan, M.; Zhang, W.; Lin, H.; Wu, S.; Lu, C.; Tang, S.; Liu, D.; Cai, J. Poly(α-l-lysine)-based nanomaterials for versatile biomedical applications: Current advances and perspectives. Bioact. Mater. 2020, 6, 1878–1909. [Google Scholar] [CrossRef]
- Stamou, A.; Iatrou, H.; Tsitsilianis, C. NIPAm-based modification of poly(l-lysine): A ph-dependent LCST-type thermo-responsive biodegradable polymer. Polymers 2022, 14, 802. [Google Scholar] [CrossRef]
- Yu, S.M.; Conticello, V.P.; Zhang, G.; Kayser, C.; Fournier, M.J.; Mason, T.L.; Tirrell, D.A. Smectic ordering in solutions and films of a rod-like polymer owing to monodispersity of chain length. Nature 1997, 389, 167–170. [Google Scholar] [CrossRef]
- Pandit, G.; Roy, K.; Agarwal, U.; Chatterjee, S. Self-assembly mechanism of a peptide-based drug delivery vehicle. ACS Omega 2018, 3, 3143–3155. [Google Scholar] [CrossRef] [PubMed]
- Locarno, S.; Argentiere, S.; Ruffoni, A.; Maggioni, D.; Soave, R.; Bucci, R.; Erba, E.; Lenardi, C.; Gelmi, M.L.; Clerici, F. Self-assembled hydrophobic Ala-Aib peptide encapsulating curcumin: A convenient system for water insoluble drugs. RSC Adv. 2020, 10, 9964–9975. [Google Scholar] [CrossRef] [PubMed]
- Doty, P.; Bradbury, J.H.; Holtzer, A.M. Polypeptides. IV. The molecular weight, configuration and association of poly-γ-benzyl-l-glutamate in various solvents. J. Am. Chem. Soc. 1956, 78, 947–954. [Google Scholar] [CrossRef]
- Brzezinska, K.R.; Deming, T.J. Synthesis of ABA triblock copolymers via acyclic diene metathesis polymerization and living polymerization of α-amino acid−N-carboxyanhydrides. Macromolecules 2001, 34, 4348–4354. [Google Scholar] [CrossRef]
- Gao, H.; Hu, Z.; Guan, Q.; Liu, Y.; Zhu, F.; Wu, Q. Synthesis and thermoreversible gelation of coil-helical polyethylene-block-poly(γ-benzyl-l-glutamate) diblock copolymer. Polymer 2013, 54, 4923–4929. [Google Scholar] [CrossRef]
- Gao, H.; Li, G.; Hu, Z.; Xiao, Z.; Liang, G.; Wu, Q. Synthesis of amphiphilic polyethylene-b-poly(l-glutamate) block copolymers with vastly different solubilities and their stimuli-responsive polymeric micelles in aqueous solution. Polymer 2014, 55, 4593–4600. [Google Scholar] [CrossRef]
- Chécot, F.; Lecommandoux, S.; Gnanou, Y.; Klok, H.-A. Water-Soluble Stimuli-Responsive Vesicles from Peptide-Based Diblock Copolymers. Angew. Chem. Int. Ed. 2002, 41, 1339–1343. [Google Scholar] [CrossRef]
- Checot, F.; Lecommandoux, S.; Klok, H.-A.; Gnanou, Y. From supramolecular polymersomes to stimuli-responsive nano-capsules based on poly(diene-b-peptide) diblock copolymers. Eur. Phys. J. E 2003, 10, 25–35. [Google Scholar] [CrossRef]
- Kukula, H.; Schlaad, H.; Antonietti, M.; Förster, S. The formation of polymer vesicles or “peptosomes” by polybutadiene-block-poly(l-glutamate)s in dilute aqueous solution. J. Am. Chem. Soc. 2002, 124, 1658–1663. [Google Scholar] [CrossRef]
- Sigel, R.; Łosik, M.; Schlaad, H. pH Responsiveness of Block Copolymer Vesicles with a Polypeptide Corona. Langmuir 2007, 23, 7196–7199. [Google Scholar] [CrossRef]
- Gebhardt, K.E.; Ahn, S.; Venkatachalam, G.; Savin, D.A. Rod-Sphere Transition in Polybutadiene−Poly(l-lysine) Block Copolymer Assemblies. Langmuir 2007, 23, 2851–2856. [Google Scholar] [CrossRef]
- Chécot, F.; Brûlet, A.; Oberdisse, J.; Gnanou, Y.; Mondain-Monval, O.; Lecommandoux, S. Structure of polypeptide-based diblock copolymers in solution: stimuli-responsive vesicles and micelles. Langmuir 2005, 21, 4308–4315. [Google Scholar] [CrossRef] [Green Version]
- Babin, J.; Rodriguez-Hernandez, J.; Lecommandoux, S.; Klok, H.-A.; Achard, M.-F. Self-assembled nanostructures from peptide–synthetic hybrid block copolymers: Complex, stimuli-responsive rod–coil architectures. Faraday Discuss. 2005, 128, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Zai, S.; Liu, F.; Gao, H.; Li, C.; Zhou, G.; Cheng, S.; Guo, L.; Zhang, L.; Zhu, F.; Wu, Q. Longstanding living polymerization of ethylene: Substituent effect on bridging carbon of 2-pyridinemethanamine nickelcatalysts. Chem. Commun. 2010, 46, 4321–4323. [Google Scholar] [CrossRef]
- Zai, S.; Gao, H.; Huang, Z.; Hu, H.; Wu, H.; Wu, Q. Substituent effects of pyridine-amine nickel catalyst precursors on ethylene polymerization. ACS Catal. 2012, 2, 433–440. [Google Scholar] [CrossRef]
- Gao, H.; Hu, H.; Zhu, F.; Wu, Q. A thermally robust amine–imine nickel catalyst precursor for living polymerization of ethylene above room temperature. Chem. Commun. 2012, 48, 3312–3314. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, L.; Gao, H.; Zhu, F.; Wu, Q. Design of thermally stable amine-imine nickel catalyst precursors for living polymerization of ethylene: Effect of ligand substituents on catalytic behavior and polymer properties. Chem. Eur. J. 2014, 20, 3225–3233. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gao, H.; Chen, D.; Li, G.; Tan, Y.; Liang, G.; Zhu, F.; Wu, Q. Ligand-Directed Regioselectivity in Amine–Imine Nickel-Catalyzed 1-Hexene Polymerization. ACS Catal. 2014, 5, 122–128. [Google Scholar] [CrossRef]
- Hu, H.; Chen, D.; Gao, H.; Zhong, L.; Wu, Q. Amine–imine palladium catalysts for living polymerization of ethylene and copolymerization of ethylene with methyl acrylate: Incorporation of acrylate units into the main chain and branch end. Polym. Chem. 2015, 7, 529–537. [Google Scholar] [CrossRef]
- Zheng, H.; Pei, L.; Deng, H.; Gao, H.; Gao, H. Electronic effects of amine-imine nickel and palladium catalysts on ethylene (co)polymerization. Eur. Polym. J. 2023, 184. [Google Scholar] [CrossRef]
- Deng, H.; Zheng, H.; Gao, H.; Pei, L.; Gao, H. Late Transition Metal Catalysts with Chelating Amines for Olefin Polymerization. Catalysts 2022, 12, 936. [Google Scholar] [CrossRef]
- Arriola, D.J.; Carnahan, E.M.; Hustad, P.D.; Kuhlman, R.L.; Wenzel, T.T. Catalytic Production of Olefin Block Copolymers via Chain Shuttling Polymerization. Science 2006, 312, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Zintl, M.; Rieger, B. Novel Olefin Block Copolymers through Chain-Shuttling Polymerization. Angew. Chem. Int. Ed. 2006, 46, 333–335. [Google Scholar] [CrossRef]
- Han, C.J.; Lee, M.S.; Byun, D.-J.; Kim, S.Y. Synthesis of Hydroxy-Terminated Polyethylene via Controlled Chain Transfer Reaction and Poly(ethylene-b-caprolactone) Block Copolymer. Macromolecules 2002, 35, 8923–8925. [Google Scholar] [CrossRef]
- Deng, C.; Rong, G.; Tian, H.; Tang, Z.; Chen, X.; Jing, X. Synthesis and characterization of poly(ethylene glycol)-b-poly (l-lactide)-b-poly(l-glutamic acid) triblock copolymer. Polymer 2005, 46, 653–659. [Google Scholar] [CrossRef]
- You, Y.; Chen, Y.; Hua, H.; Dong, C. Synthesis and thermoreversible gelation of dendron-like polypeptide/linear poly(ε-caprolactone)/dendron-like polypeptide triblock copolymers. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 709–718. [Google Scholar] [CrossRef]
- Bonduelle, C. Secondary structures of synthetic polypeptide polymers. Polym. Chem. 2017, 9, 1517–1529. [Google Scholar] [CrossRef]
- Canalp, M.B.; Binder, W.H. Hybrid polymers bearing oligo-l-lysine(carboxybenzyl)s: Synthesis and investigations of secondary structure. RSC Adv. 2020, 10, 1287–1295. [Google Scholar] [CrossRef] [Green Version]
- Radchenko, A.V.; Grange, J.; Vax, A.; Jean-Baptiste-dit-Dominique, F.; Matmour, R.; Grelier, S.; Peruch, F. Facile synthesis of 1,4-cis-polyisoprene–polypeptide hybrids with different architectures. Polym. Chem. 2019, 10, 2456–2468. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, J.; Zhang, P.; Luan, X.; Mai, Y. “Rod–coil” copolymers get self-assembled in solution. Mater. Chem. Front. 2019, 3, 2283–2307. [Google Scholar] [CrossRef]
- Song, Z.; Tan, Z.; Cheng, J. Recent Advances and Future Perspectives of Synthetic Polypeptides from N-Carboxyanhydrides. Macromolecules 2019, 52, 8521–8539. [Google Scholar] [CrossRef]
- Wu, D.; Huang, Y.; Xu, F.; Mai, Y.; Yan, D. Recent advances in the solution self-assembly of amphiphilic “rod-coil” copolymers. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1459–1477. [Google Scholar] [CrossRef] [Green Version]
- Gohy, J.-F.; Willet, N.; Varshney, S.; Zhang, J.-X.; Jérôme, R. Core-Shell-Corona Micelles with a Responsive Shell. Angew. Chem. Int. Ed. 2001, 40, 3214–3216. [Google Scholar] [CrossRef]











| Entry | Copolymer b | Copolymer Precursor | NCA/PE c | Mn-PLLd (kg/mol) | Mnd (kg/mol) | PDI e |
|---|---|---|---|---|---|---|
| 1 | PE607-b-PLL97 | PE607-b-PZL97 | 100 | 12.5 | 29.5 | 1.3 |
| 2 | PE607-b-PLL188 | PE607-b-PZL188 | 200 | 24.3 | 41.3 | 1.4 |
| 3 | PE607-b-PLL275 | PE607-b-PZL275 | 300 | 35.5 | 52.5 | 1.4 |
| Entry | Copolymer | PE/PGA b Wt% | CMC c (mg/mL) | Rh d (nm) | R e (nm) |
|---|---|---|---|---|---|
| 1 | PE607-b-PLL97 | 58/42 | 0.016 | 141 ± 3 | - |
| 2 | PE607-b-PLL188 | 41/59 | 0.025 | 126 ± 2 | - |
| 3 | PE607-b-PLL275 | 32/68 | 0.040 | 117 ± 2 | 110 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, L.; Ma, H.; Jiang, Y.; Zheng, H.; Gao, H. Amphiphilic Polyethylene-b-poly(L-lysine) Block Copolymer: Synthesis, Self-Assembly, and Responsivity. Int. J. Mol. Sci. 2023, 24, 5495. https://doi.org/10.3390/ijms24065495
Pei L, Ma H, Jiang Y, Zheng H, Gao H. Amphiphilic Polyethylene-b-poly(L-lysine) Block Copolymer: Synthesis, Self-Assembly, and Responsivity. International Journal of Molecular Sciences. 2023; 24(6):5495. https://doi.org/10.3390/ijms24065495
Chicago/Turabian StylePei, Lixia, Hongyu Ma, Yan Jiang, Handou Zheng, and Haiyang Gao. 2023. "Amphiphilic Polyethylene-b-poly(L-lysine) Block Copolymer: Synthesis, Self-Assembly, and Responsivity" International Journal of Molecular Sciences 24, no. 6: 5495. https://doi.org/10.3390/ijms24065495