Quercetin 3-O-(6″-O-E-caffeoyl)-β-D-glucopyranoside, a Flavonoid Compound, Promotes Melanogenesis through the Upregulation of MAPKs and Akt/GSK3β/β-Catenin Signaling Pathways
Abstract
:1. Introduction
2. Results
2.1. Toxicity Test of the CC7 on B16 Cells
2.2. Melanogenic Effect of the CC7 on B16 Cells and PIG3V Melanocytes
2.3. CC7 Promotes the Protein Expression Levels of MITF and TRPs in B16 Cells
2.4. CC7 Promotes the Protein Expression Levels of p38MAPKs and JNK in B16 Cells
2.5. CC7 Enhances the Melanogenesis Not Affected by MAPKs in B16 Cells
2.6. CC7 Promotes the Protein Expression Levels of Akt, GSK3β, and β-Catenin in B16 Cells
2.7. Melanogenic Effect of CC7 Involves Upregulating the Akt/GSK3β/β-Catenin in B16 Cells
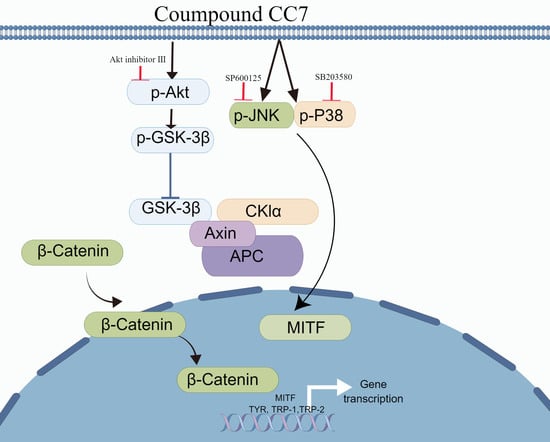
3. Discussion
4. Materials and Methods
4.1. Drug Preparation and Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Tyrosinase Activity
4.5. Melanin Contents Measurement
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 8-MOP | 8-methoxypsoralan |
| TYR | Tyrosinase |
| TRP-1 | Tyrosinase-related protein-1 |
| TRP-2 | Tyrosinase-related protein-2 |
| MITF | microphthalmia-associated transcription factor |
| MAPK | mitogen-activated protein kinase |
| JNK | c-Jun N-terminal kinase |
| ERK | extracellular-related kinase |
| Akt | Protein kinase B |
| GSK3β | Glycogen Synthase Kinase 3 Beta |
Appendix A
| C Atom | δC | δH (J/Hz) |
|---|---|---|
| 2 | 155.81 | |
| 3 | 133.35 | |
| 4 | 177.25 | |
| 5 | 161.12 | |
| 6 | 99.09 | 6.12, d (2.1) |
| 7 | 165.19 | |
| 8 | 93.69 | |
| 9 | 156.35 | |
| 10 | 103.39 | |
| 1′ | 121.09 | |
| 2′ | 115.82 | 7.52, d (2.2) |
| 3′ | 144.87 | |
| 4′ | 148.45 | |
| 5′ | 115.19 | 6.82, d (8.5) |
| 6′ | 121.80 | 7.64, dd (8.5,2.2) |
| 1″ | 101.52 | 5.40, d (7.8) |
| 2″ | 71.01 | 3.59, dd (9.5, 7.8) |
| 3″ | 72.93 | 3.43, dd (9.5, 3.3) |
| 4″ | 68.27 | 3.67, m |
| 5″ | 72.93 | 3.67, m |
| 6″ | 63.08 | 4.02, dd (11.3, 4.8) |
| 4.12, dd (11.3, 4.8) | ||
| 1‴ | 125.32 | |
| 2‴ | 114.57 | 6.96, d (2.1) |
| 3‴ | 145.71 | |
| 4‴ | 148.55 | |
| 5‴ | 115.57 | 6.74, d (8.1) |
| 6‴ | 121.29 | 6.80, dd (8.1, 2.1) |
| 7‴ | 145.26 | 7.31, d (15.9) |
| 8‴ | 113.26 | 6.00, d (15.9) |
| 9‴ | 166.22 |
Appendix B

References
- Zhou, S.; Zeng, H.; Huang, J.; Lei, L.; Tong, X.; Li, S.; Zhou, Y.; Guo, H.; Khan, M.; Luo, L.; et al. Epigenetic regulation of melanogenesis. Ageing Res. Rev. 2021, 69, 101349. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zeng, H.L.; Wen, X.Y.; Jiang, L.; Fu, C.H.; Hu, Y.B.; Lei, X.-X.; Zhang, L.; Yu, X.; Yang, S.-Y.; et al. Selaginellin Inhibits Melanogenesis via the MAPK Signaling Pathway. J. Nat. Prod. 2022, 85, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Gryn-Rynko, A.; Sperkowska, B.; Majewski, M.S. Screening and Structure-Activity Relationship for Selective and Potent Anti-Melanogenesis Agents Derived from Species of Mulberry (Genus Morus). Molecules 2022, 27, 9011. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Yoon, J.H.; Youn, K.; Jun, M. Decursin prevents melanogenesis by suppressing MITF expression through the regulation of PKA/CREB, MAPKs, and PI3K/Akt/GSK-3β cascades. Biomed. Pharmacother. 2022, 147, 112651. [Google Scholar] [CrossRef] [PubMed]
- Merecz-Sadowska, A.; Sitarek, P.; Kowalczyk, T.; Zajdel, K.; Kucharska, E.; Zajdel, R. The Modulation of Melanogenesis in B16 Cells Upon Treatment with Plant Extracts and Isolated Plant Compounds. Molecules 2022, 27, 4360. [Google Scholar] [CrossRef]
- Sevilla, A.; Chéret, J.; Lee, W.; Paus, R. Concentration-dependent stimulation of melanin production as well as melanocyte and keratinocyte proliferation by melatonin in human eyelid epidermis. Exp. Dermatol. 2023, 20, 1564–1566. [Google Scholar] [CrossRef]
- Nimkar, P.; Wanjari, A. Vitiligo and the Role of Newer Therapeutic Modalities. Cureus 2022, 14, e31022. [Google Scholar] [CrossRef]
- Kumari, S.; Thng, S.T.G.; Verma, N.K.; Gautam, H.K. Melanogenesis Inhibitors. Acta Derm. Venereol. 2018, 98, 924–931. [Google Scholar] [CrossRef] [Green Version]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Tan, Z.; Deng, J.; Ye, Q.; Zhang, Z. The Antibacterial Activity of Natural-derived Flavonoids. Curr. Top. Med. Chem. 2022, 22, 1009–1019. [Google Scholar] [CrossRef]
- Calis, Z.; Mogulkoc, R.; Baltaci, K. The Roles of Flavonols/Flavonoids in Neurodegeneration and Neuroinflammation. Mini Rev. Med. Chem. 2020, 20, 1475–1488. [Google Scholar] [CrossRef]
- Park, M.Y.; Kim, Y.; Ha, S.E.; Kim, H.H.; Bhosale, P.B.; Abusaliya, A.; Jeong, S.H.; Kim, G.S. Function and Application of Flavonoids in the Breast Cancer. Int. J. Mol. Sci. 2022, 23, 7732. [Google Scholar] [CrossRef]
- Oršolić, N. Allergic Inflammation: Effect of Propolis and Its Flavonoids. Molecules 2022, 27, 6694. [Google Scholar] [CrossRef]
- Jafarinia, M.; Hosseini, M.S.; Kasiri, N.; Fazel, N.; Fathi, F.; Hakemi, M.G.; Eskandari, N. Quercetin with the potential effect on allergic diseases. Allergy Asthma Clin. Immunol. 2020, 16, 36. [Google Scholar] [CrossRef]
- Dai, C.-Y.; Liao, P.-R.; Zhao, M.-Z.; Gong, C.; Dang, Y.; Qu, Y.; Qiu, L.-S. Optimization of Ultrasonic Flavonoid Extraction from Saussurea involucrate, and the Ability of Flavonoids to Block Melanin Deposition in Human Melanocytes. Molecules 2020, 25, 313. [Google Scholar] [CrossRef] [Green Version]
- Poyedinok, N.; Mykhaylova, O.; Sergiichuk, N.; Tugay, T.; Tugay, A.; Lopatko, S.; Matvieieva, N. Effect of Colloidal Metal Nanoparticles on Biomass, Polysaccharides, Flavonoids, and Melanin Accumulation in Medicinal Mushroom Inonotus obliquus (Ach.:Pers.) Pilát. Appl. Biochem. Biotechnol. 2020, 191, 1315–1325. [Google Scholar] [CrossRef]
- Netcharoensirisuk, P.; Abrahamian, C.; Tang, R.; Chen, C.C.; Rosato, A.S.; Beyers, W.; Chao, Y.K.; Filippini, A.; Di Pietro, S.; Bartel, K.; et al. Flavonoids increase melanin production and reduce proliferation, migration and invasion of melanoma cells by blocking endolysosomal/melanosomal TPC2. Sci. Rep. 2021, 11, 8515. [Google Scholar] [CrossRef]
- Lv, J.; Fu, Y.; Cao, Y.; Jiang, S.; Yang, Y.; Song, G.; Yun, C.; Gao, R. Isoliquiritigenin inhibits melanogenesis, melanocyte dendricity and melanosome transport by regulating ERK-mediated MITF degradation. Exp. Dermatol. 2020, 29, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Feng, D.; Fang, Z.; Zhang, P. The melanin inhibitory effect of plants and phytochemicals: A systematic review. Phytomedicine 2022, 107, 154449. [Google Scholar] [CrossRef]
- Uto, T.; Ohta, T.; Katayama, K.; Shoyama, Y. Silibinin promotes melanogenesis through the PKA and p38 MAPK signaling pathways in melanoma cells. Biomed. Res. 2022, 43, 31–39. [Google Scholar] [CrossRef]
- Calzada, F.; Cedillo-Rivera, R.; Mata, R. Antiprotozoal activity of the constituents of Conyza filaginoides. J. Nat. Prod. 2001, 64, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Hopkin, A.S.; Paterson, E.K.; Ruiz, R.; Ganesan, A.K. Pigment Production Analysis in Human Melanoma Cells. Methods Mol. Biol. 2016, 359, 1–12. [Google Scholar]
- Fujii, T.; Ikeda, K.; Saito, M. Inhibitory effect of rose hip (Rosa canina L.) on melanogenesis in mouse melanoma cells and on pigmentation in brown guinea pigs. Biosci. Biotechnol. Biochem. 2011, 75, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Guo, L.; Sun, Y.; Zhou, J.; Gu, Y.; Li, Y. Baicalein inhibits melanogenesis through activation of the ERK signaling pathway. Int. J. Mol. Med. 2010, 25, 923–927. [Google Scholar] [CrossRef] [Green Version]
- Drira, R.; Sakamoto, K. Isosakuranetin, a 4'-O-methylated flavonoid, stimulates melanogenesis in B16BL6 murine melanoma cells. Life Sci. 2015, 143, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Yu-Chun, H.; Chao-Hsun, Y.; Yi-Ling, C. Citrus flavanone naringenin enhances melanogenesis through the activation of Wnt/β-catenin signalling in mouse melanoma cells. Phytomedicine 2011, 18, 1244–1249. [Google Scholar]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure and Function of Human Tyrosinase and Tyrosinase-Related Proteins. Chemistry 2018, 24, 47–55. [Google Scholar] [CrossRef]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Invest. Dermatol. 2007, 127, 751–761. [Google Scholar] [CrossRef] [Green Version]
- Aoki, H.; Moro, O. Involvement of microphthalmia-associated transcription factor (MITF) in expression of human melanocortin-1 receptor (MC1R). Life Sci. 2002, 71, 2171–2179. [Google Scholar] [CrossRef]
- Chung, Y.C.; Kim, S.; Kim, J.H.; Lee, G.S.; Lee, J.N.; Lee, N.H.; Hyun, C.G. Pratol, an O-Methylated Flavone, Induces Melanogenesis in B16F10 Melanoma Cells via p-p38 and p-JNK Upregulation. Molecules 2017, 22, 1704. [Google Scholar] [CrossRef]
- Choi, M.R.; Lee, H.; Kim, H.K.; Han, J.; Seol, J.E.; Vasileva, E.A.; Mishchenko, N.P.; Fedor eyev, S.A.; Stonik, V.A.; Ju, W.S.; et al. Echinochrome A Inhibits Melanogenesis in B16F10 Cells by Downregulating CREB Signaling. Mar. Drugs 2022, 20, 555. [Google Scholar] [CrossRef]
- Lee, Y.; Hyun, C.-G. Mechanistic Insights into the Ameliorating Effect of Melanogenesis of Psoralen Derivatives in B16F10 Melanoma Cells. Molecules 2022, 27, 2613. [Google Scholar] [CrossRef]
- Zhao, L.-M.; Han, L.-N.; Ren, F.-Z.; Chen, S.-H.; Liu, L.-H.; Wang, M.-X.; Shan, B.E. An ester extract of Cochinchina momordica seeds induces differentiation of melanoma B16 F1 cells via MAPKs signaling. Asian Pac. J. Cancer Prev. 2012, 13, 3795–3802. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Pan, W. GSK3: A multifaceted kinase in Wnt signaling. Trends Biochem. Sci. 2010, 35, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Bienz, M. beta-Catenin: A pivot between cell adhesion and Wnt signalling. Curr. Biol. 2005, 15, R64–R67. [Google Scholar] [CrossRef] [Green Version]
- Bellei, B.; Flori, E.; Izzo, E.; Maresca, V.; Picardo, M. GSK3beta inhibition promotes melanogenesis in mouse B16 melanoma cells and normal human melanocytes. Cell Signal 2008, 20, 1750–1761. [Google Scholar] [CrossRef]
- Niu, C.; Yin, L.; Aisa, H.A. Novel Furocoumarin Derivatives Stimulate Melanogenesis in B16 Melanoma Cells by Up-Regulation of MITF and TYR Family via Akt/GSK3β/β-Catenin Signaling Pathways. Int. J. Mol. Sci. 2018, 19, 746. [Google Scholar] [CrossRef] [Green Version]
- Zang, D.; Niu, C.; Aisa, H.A. Amine derivatives of furocoumarin induce melanogenesis by activating Akt/GSK-3β/β-catenin signal pathway. Drug Des. Devel. Ther. 2019, 13, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Elsbaey, M.; Sallam, A.; El-Metwally, M.; Nagata, M.; Tanaka, C.; Shimizu, K.; Miyamoto, T. Miyamoto. Melanogenesis Inhibitors from the Endophytic Fungus Aspergillus amstelodami . Chem. Biodivers 2019, 16, e1900237. [Google Scholar] [CrossRef]
- Win, N.N.; Woo, S.-Y.; Ngwe, H.; Wong, C.P.; Ito, T.; Okamoto, Y.; Tanaka, M.; Imagawa, H.; Asakawa, Y.; Abe, I.; et al. Tetrahydrofuran lignans: Melanogenesis inhibitors from Premna integrifolia wood collected in Myanmar. Fitoterapia 2018, 127, 308–313. [Google Scholar] [CrossRef]
- Hałdys, K.; Goldeman, W.; Jewgiński, M.; Wolińska, E.; Anger-Góra, N.; Rossowska, J.; Latajka, R. Halogenated aromatic thiosemicarbazones as potent inhibitors of tyrosinase and melanogenesis. Bioorg. Chem. 2020, 94, 103419. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Noh, S.G.; Ryu, I.Y.; Park, C.; Lee, J.Y.; Chun, P.; Moon, H.R.; Chung, H.Y. (E)-1-(Furan-2-yl)-(substituted phenyl)prop-2-en-1-one Derivatives as Tyrosinase Inhibitors and Melanogenesis Inhibition: An In Vitro and In Silico Study. Molecules 2020, 25, 5460. [Google Scholar] [CrossRef] [PubMed]
- Kenji, O.C.; Yukihiro, A.; Yoshinori, N. Stimulation of melanogenesis by the citrus flavonoid naringenin in mouse B16 melanoma cells. Biosci. Biotechnol. Biochem. 2006, 70, 1499–1501. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Nueraihemaiti, M.; Zang, D.; Edirs, S.; Zou, G.; Aisa, H.A. Quercetin 3-O-(6″-O-E-caffeoyl)-β-D-glucopyranoside, a Flavonoid Compound, Promotes Melanogenesis through the Upregulation of MAPKs and Akt/GSK3β/β-Catenin Signaling Pathways. Int. J. Mol. Sci. 2023, 24, 4780. https://doi.org/10.3390/ijms24054780
Liu C, Nueraihemaiti M, Zang D, Edirs S, Zou G, Aisa HA. Quercetin 3-O-(6″-O-E-caffeoyl)-β-D-glucopyranoside, a Flavonoid Compound, Promotes Melanogenesis through the Upregulation of MAPKs and Akt/GSK3β/β-Catenin Signaling Pathways. International Journal of Molecular Sciences. 2023; 24(5):4780. https://doi.org/10.3390/ijms24054780
Chicago/Turabian StyleLiu, Changhai, Mayire Nueraihemaiti, Deng Zang, Salamet Edirs, Guoan Zou, and Haji Akber Aisa. 2023. "Quercetin 3-O-(6″-O-E-caffeoyl)-β-D-glucopyranoside, a Flavonoid Compound, Promotes Melanogenesis through the Upregulation of MAPKs and Akt/GSK3β/β-Catenin Signaling Pathways" International Journal of Molecular Sciences 24, no. 5: 4780. https://doi.org/10.3390/ijms24054780