Artificial Diets with Altered Levels of Sulfur Amino Acids Induce Anticancer Activity in Mice with Metastatic Colon Cancer, Ovarian Cancer and Renal Cell Carcinoma
Abstract
:1. Introduction
2. Results
2.1. Screening of Methionine-Deficient Diets Suplemented with Cysteine and Taurine in Mice with Ovarian Cancer
2.2. Diets B1 and B2B Induce Moderate Anticancer Activity in Mice with Renal Cell Carcinoma
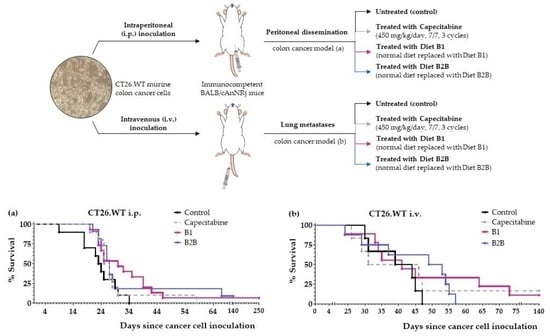
2.3. Diets B1 and B2B Induce Marked Anticancer Activity in Mice with Metastatic Colon Cancer
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture Conditions
4.2. Drugs and Reagents
4.3. Diets Preparation and Composition
4.4. Animals
4.5. In Vivo Cancer Models
4.6. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. The Warburg Effect: Why and How Do Cancer Cells Activate Glycolysis in the Presence of Oxygen? Anticancer. Agents Med. Chem. 2008, 8, 305–312. [Google Scholar] [CrossRef]
- Tajan, M.; Vousden, K.H. Dietary Approaches to Cancer Therapy. Cancer Cell 2020, 37, 767–785. [Google Scholar] [CrossRef]
- Kanarek, N.; Petrova, B.; Sabatini, D.M. Dietary modifications for enhanced cancer therapy. Nature 2020, 579, 507–517. [Google Scholar] [CrossRef]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Gao, X.; Sanderson, S.M.; Dai, Z.; Reid, M.A.; Cooper, D.E.; Lu, M.; Richie, J.P., Jr.; Ciccarella, A.; Calcagnotto, A.; Mikhael, P.G.; et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 2019, 572, 397–401. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.L.; Wu, D.Z.; Yuan, Y.W.; Xin, L. Methionine restriction enhances the chemotherapeutic sensitivity of colorectal cancer stem cells by miR-320d/c-Myc axis. Mol. Cell. Biochem. 2022, 477, 2001–2013. [Google Scholar] [CrossRef]
- Li, T.; Tan, Y.-T.; Chen, Y.-X.; Zheng, X.-J.; Wang, W.; Liao, K.; Mo, H.-Y.; Lin, J.; Yang, W.; Piao, H.-L.; et al. Methionine deficiency facilitates antitumour immunity by altering m 6 A methylation of immune checkpoint transcripts. Gut 2022, 0, 501–511. [Google Scholar] [CrossRef]
- Komninou, D.; Leutzinger, Y.; Reddy, B.S.; Richie, J.P. Methionine restriction inhibits colon carcinogenesis. Nutr. Cancer 2006, 54, 202–208. [Google Scholar] [CrossRef]
- Jiménez-Alonso, J.J.; Guillén-Mancina, E.; Calderón-Montaño, J.M.; Jiménez-González, V.; Díaz-Ortega, P.; Burgos-Morón, E.; López-Lázaro, M. Artificial Diets Based on Selective Amino Acid Restriction versus Capecitabine in Mice with Metastatic Colon Cancer. Nutrients 2022, 14, 3378. [Google Scholar] [CrossRef]
- Zhang, T.; Bauer, C.; Newman, A.C.; Uribe, A.H.; Athineos, D.; Blyth, K.; Maddocks, O.D.K. Polyamine pathway activity promotes cysteine essentiality in cancer cells. Nat. Metab. 2020, 2, 1062–1076. [Google Scholar] [CrossRef]
- Wu, J.; Yeung, S.-C.J.; Liu, S.; Qdaisat, A.; Jiang, D.; Liu, W.; Cheng, Z.; Liu, W.; Wang, H.; Li, L.; et al. Cyst(e)ine in nutrition formulation promotes colon cancer growth and chemoresistance by activating mTORC1 and scavenging ROS. Signal Transduct. Target. Ther. 2021, 6, 188. [Google Scholar] [CrossRef]
- Maddocks, O.D.K.; Berkers, C.R.; Mason, S.M.; Zheng, L.; Blyth, K.; Gottlieb, E.; Vousden, K.H. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 2013, 493, 542–546. [Google Scholar] [CrossRef]
- Humpton, T.J.; Hock, A.K.; Maddocks, O.D.K.; Vousden, K.H. p53-mediated adaptation to serine starvation is retained by a common tumour-derived mutant. Cancer Metab. 2018, 6, 18. [Google Scholar] [CrossRef]
- Tajan, M.; Hennequart, M.; Cheung, E.C.; Zani, F.; Hock, A.K.; Legrave, N.; Maddocks, O.D.K.; Ridgway, R.A.; Athineos, D.; Suárez-Bonnet, A.; et al. Serine synthesis pathway inhibition cooperates with dietary serine and glycine limitation for cancer therapy. Nat. Commun. 2021, 12, 366. [Google Scholar] [CrossRef]
- Pranzini, E.; Pardella, E.; Muccillo, L.; Leo, A.; Nesi, I.; Santi, A.; Parri, M.; Zhang, T.; Uribe, A.H.; Lottini, T.; et al. SHMT2-mediated mitochondrial serine metabolism drives 5-FU resistance by fueling nucleotide biosynthesis. Cell Rep. 2022, 40, 111233. [Google Scholar] [CrossRef]
- Gravel, S.-P.; Hulea, L.; Toban, N.; Birman, E.; Blouin, M.-J.; Zakikhani, M.; Zhao, Y.; Topisirovic, I.; St-Pierre, J.; Pollak, M. Serine Deprivation Enhances Antineoplastic Activity of Biguanides. Cancer Res. 2014, 74, 7521–7533. [Google Scholar] [CrossRef]
- Muthusamy, T.; Cordes, T.; Handzlik, M.K.; You, L.; Lim, E.W.; Gengatharan, J.; Pinto, A.F.M.; Badur, M.G.; Kolar, M.J.; Wallace, M.; et al. Serine restriction alters sphingolipid diversity to constrain tumour growth. Nature 2020, 586, 790–795. [Google Scholar] [CrossRef]
- Yeatman, T.J.; Risley, G.L.; Brunson, M.E. Depletion of Dietary Arginine Inhibits Growth of Metastatic Tumor. Arch. Surg. 1991, 126, 1376–1382. [Google Scholar] [CrossRef]
- Alexandrou, C.; Al-Aqbi, S.S.; Higgins, J.A.; Boyle, W.; Karmokar, A.; Andreadi, C.; Luo, J.L.; Moore, D.A.; Viskaduraki, M.; Blades, M.; et al. Sensitivity of Colorectal Cancer to Arginine Deprivation Therapy is Shaped by Differential Expression of Urea Cycle Enzymes. Sci. Rep. 2018, 8, 12096. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Guillén-Mancina, E.; José Jiménez-Alonso, J.J.; Jiménez-González, V.; Burgos-Morón, E.; Mate, A.; Concepción Pérez-Guerrero, M.; López-Lázaro, M. Manipulation of Amino Acid Levels with Artificial Diets Induces a Marked Anticancer Activity in Mice with Renal Cell Carcinoma. Int. J. Mol. Sci. 2022, 2022, 16132. [Google Scholar] [CrossRef]
- López-Lázaro, M. Selective amino acid restriction therapy (SAART): A non- pharmacological strategy against all types of cancer cells. Oncoscience 2015, 2, 857. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136. [Google Scholar] [CrossRef]
- Zhang, N. Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Anim. Nutr. 2018, 4, 11–16. [Google Scholar] [CrossRef]
- Weber, R.; Birsoy, K. The Transsulfuration Pathway Makes, the Tumor Takes. Cell Metab. 2019, 30, 845–846. [Google Scholar] [CrossRef]
- Yin, J.; Ren, W.; Yang, G.; Duan, J.; Huang, X.; Fang, R.; Li, C.; Li, T.; Yin, Y.; Hou, Y.; et al. l-Cysteine metabolism and its nutritional implications. Mol. Nutr. Food Res. 2016, 60, 134–146. [Google Scholar] [CrossRef]
- Combs, J.A.; DeNicola, G.M. The Non-Essential Amino Acid Cysteine Becomes Essential for Tumor Proliferation and Survival. Cancers 2019, 11, 678. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Klein Geltink, R.I.; Parker, S.J.; Sorensen, P.H. Transsulfuration, minor player or crucial for cysteine homeostasis in cancer. Trends Cell Biol. 2022, 32, 800–814. [Google Scholar] [CrossRef]
- Szabõ, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef]
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–164. [Google Scholar] [CrossRef]
- Ma, N.; He, F.; Kawanokuchi, J.; Wang, G.; Yamashita, T. Taurine and Its Anticancer Functions: In Vivo and In Vitro Study. Adv. Exp. Med. Biol. 2022, 1370, 121–128. [Google Scholar] [CrossRef]
- Vettore, L.; Westbrook, R.L.; Tennant, D.A. New aspects of amino acid metabolism in cancer. Br. J. Cancer 2020, 122, 150–156. [Google Scholar] [CrossRef]
- Mates, J.M.; Segura, J.A.; Alonso, F.J.; Marquez, J. Sulphur-containing non enzymatic antioxidants: Therapeutic tools against cancer. Front. Biosci. - Sch. 2012, 4, 722–748. [Google Scholar] [CrossRef]
- Burg, M.B.; Ferraris, J.D. Intracellular Organic Osmolytes: Function and Regulation. J. Biol. Chem. 2008, 283, 7309–7313. [Google Scholar] [CrossRef]
- Park, J.H.; Han, Q.; Zhao, M.; Tan, Y.; Higuchi, T.; Yoon, S.N.; Sugisawa, N.; Yamamoto, J.; Bouvet, M.; Clary, B.; et al. Oral Recombinant Methioninase Combined with Oxaliplatinum and 5-Fluorouracil Regressed a Colon Cancer Growing on the Peritoneal Surface in a Patient-Derived Orthotopic Xenograft Mouse Model. Tissue Cell 2019, 61, 109–114. [Google Scholar] [CrossRef]
- Sanderson, S.M.; Gao, X.; Dai, Z.; Locasale, J.W. Methionine Metabolism in Health and Cancer: A Nexus of Diet and Precision Medicine. Nat. Rev. Cancer 2019, 19, 625–637. [Google Scholar] [CrossRef]
- Kaiser, P. Methionine dependence of cancer. Biomolecules 2020, 10, 568. [Google Scholar] [CrossRef]
- Kubota, Y.; Han, Q.; Hamada, K.; Aoki, Y.; Masaki, N.; Obara, K.; Tsunoda, T.; Hoffman, R.M. Long-term Stable Disease in a Rectal-cancer Patient Treated by Methionine Restriction With Oral Recombinant Methioninase and a Low-methionine Diet. Anticancer Res. 2022, 42, 3857–3861. [Google Scholar] [CrossRef]
- Tan, Y.; Sun, X.; Xu, M.; Tan, X.; Sasson, A.; Rashidi, B.; Han, Q.; Tan, X.; Wang, X.; An, Z.; et al. Efficacy of recombinant methioninase in combination with cisplatin on human colon tumors in nude mice. Clin. Cancer Res. 1999, 5, 2157–2163. [Google Scholar]
- Bonifácio, V.D.B.; Pereira, S.A.; Serpa, J.; Vicente, J.B. Cysteine metabolic circuitries: Druggable targets in cancer. Br. J. Cancer 2021, 124, 862–879. [Google Scholar] [CrossRef]
- Walton, J.B.; Farquharson, M.; Mason, S.; Port, J.; Kruspig, B.; Dowson, S.; Stevenson, D.; Murphy, D.; Matzuk, M.; Kim, J.; et al. CRISPR/Cas9-derived models of ovarian high grade serous carcinoma targeting Brca1, Pten and Nf1, and correlation with platinum sensitivity. Sci. Rep. 2017, 7, 16827. [Google Scholar] [CrossRef]
- Vasey, P.A.; McMahon, L.; Paul, J.; Reed, N.; Kaye, S.B. A phase II trial of capecitabine (Xeloda®) in recurrent ovarian cancer. Br. J. Cancer 2003, 89, 1843–1848. [Google Scholar] [CrossRef]
- Guillén-Mancina, E.; Jiménez-Alonso, J.J.; Calderón-Montaño, J.M.; Jiménez-González, V.; Díaz-Ortega, P.; Burgos-Morón, E.; López-Lázaro, M. Artificial Diets with Selective Restriction of Amino Acids and Very Low Levels of Lipids Induce Anticancer Activity in Mice with Metastatic Triple-Negative Breast Cancer. Preprints 2022, 2022120399. [Google Scholar] [CrossRef]
- Yue, T.; Li, J.; Zhu, J.; Zuo, S.; Wang, X.; Liu, Y.; Liu, J.; Liu, X.; Wang, P.; Chen, S. Hydrogen sulfide creates a favorable immune microenvironment for colon cancer. Cancer Res. 2022, 83, 595–612. [Google Scholar] [CrossRef]
- Dilek, N.; Papapetropoulos, A.; Toliver-Kinsky, T.; Szabo, C. Hydrogen sulfide: An endogenous regulator of the immune system. Pharmacol. Res. 2020, 161, 105119. [Google Scholar] [CrossRef]
- Schuller-Levis, G.B.; Park, E. Taurine and Its Chloramine: Modulators of Immunity. Neurochem. Res. 2004, 29, 117–126. [Google Scholar] [CrossRef]
- Muir, A.; Danai, L.V.; Gui, D.Y.; Waingarten, C.Y.; Lewis, C.A.; Vander Heiden, M.G. Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition. Elife 2017, 6, e27713. [Google Scholar] [CrossRef]
- Liu, L.; Liu, R.; Liu, Y.; Li, G.; Chen, Q.; Liu, X.; Ma, S. Cystine-glutamate antiporter xCT as a therapeutic target for cancer. Cell Biochem. Funct. 2021, 39, 174–179. [Google Scholar] [CrossRef]
- Kolinsky, K.; Shen, B.-Q.Q.; Zhang, Y.-E.E.; Kohles, J.; Dugan, U.; Zioncheck, T.F.; Heimbrook, D.; Packman, K.; Higgins, B. In vivo activity of novel capecitabine regimens alone and with bevacizumab and oxaliplatin in colorectal cancer xenograft models. Mol. Cancer Ther. 2009, 8, 75–82. [Google Scholar] [CrossRef]
- Griswold, D.P.; Corbett, T.H. A colon tumor model for anticancer agent evaluation. Cancer 2006, 36, 2441–2444. [Google Scholar] [CrossRef]
- Wang, L.; Hu, X.; Xu, Y.; Liu, Z. Arsenic trioxide inhibits lung metastasis of mouse colon cancer via reducing the infiltration of regulatory T cells. Tumor Biol. 2016, 37, 15165–15173. [Google Scholar] [CrossRef]




| Treatment | Survival Time (n = 3; Days) | Survival Time (Mean ± SEM; Days) | Survival Improvement vs. Control (Days) | p-Value vs. Control | ||
|---|---|---|---|---|---|---|
| Control | 49 | 45 | 48 | 47.3 ± 1.2 | 0.0 | - |
| Capecitabine | 48 | 49 | 49 | 48.7 ± 0.3 | +1.3 | 0.3458 |
| Diet B1 | 56 | 59 | 63 | 59.3 ± 2.0 | +12.0 | 0.0339 |
| Diet B1A | 57 | 55 | 57 | 56.3 ± 0.7 | +9.0 | 0.0339 |
| Diet B1B | 57 | 56 | 58 | 57.0 ± 0.6 | +9.7 | 0.0339 |
| Diet B2 | 57 | 52 | 57 | 55.3 ± 1.7 | +8.0 | 0.0339 |
| Diet B2A | 51 | 62 | 55 | 56.0 ± 3.2 | +8.7 | 0.0339 |
| Diet B2B | 62 | 58 | 55 | 58.3 ± 2.0 | +11.0 | 0.0339 |
| Treatment | n | Survival Time (Mean ± SEM; Days) | Survival Improvement vs. Control (Days) | p-Value vs. Control |
|---|---|---|---|---|
| Control | 3 | 21.7 ± 3.8 | - | - |
| Sunitinib | 3 | 46.7 ± 5.4 | +25.0 | 0.0339 |
| B1 | 6 | 29.3 ± 1.6 | +7.7 | 0.0833 |
| B2B | 6 | 30.3 ± 3.0 | +8.7 | 0.1573 |
| Treatment | n | Survival Time (Mean ± SEM; Days) | Survival Improvement vs. Control (Days) | p-Value vs. Control |
|---|---|---|---|---|
| Control | 6 | 39.3 ± 3.0 | - | - |
| Capecitabine | 6 | 53.2 ± 17.7 | +13.8 | 1.0000 |
| B1 | 9 | 54.3 ± 11.9 | +15.0 | 0.5964 |
| B2B | 8 | 44.8 ± 4.6 | +5.4 | 0.2914 |
| Treatment | n | Survival Time (Mean ± SEM; days) | Survival Improvement vs. Control (days) | p-Value vs. Control |
|---|---|---|---|---|
| Control | 10 | 23.2 ± 2.3 | - | - |
| Capecitabine | 10 | 28.8 ± 3.4 | +5.6 | 0.2068 |
| B1 | 15 | 45.1 ± 14.8 | +21.9 | 0.0279 |
| B1+Met | 3 | 26.7 ± 2.0 | +3.5 | 0.4300 |
| B2B | 11 | 39.8 ± 10.7 | +16.6 | 0.1418 |
| B2B+Met | 5 | 23.8 ± 2.1 | +0.6 | 0.9040 |
| DIET | TC5 | B1 | B1A | B1B | B2 | B2A | B2B | B1 + Met | B2B + Met |
|---|---|---|---|---|---|---|---|---|---|
| Casein | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| Glutamine | 5.0 | - | - | - | 5.0 | 5.0 | 5.0 | - | 5.0 |
| Leucine | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Cystine | - | 0.2 | 0.2 | - | 0.2 | 0.2 | - | 0.2 | - |
| Taurine | - | - | 0.2 | 0.2 | - | 0.2 | 0.2 | - | 0.2 |
| Methionine | - | - | - | - | - | - | - | 0.5 | 0.5 |
| Salmon oil | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Choline | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Vitamin Mix | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Mineral Mix | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Sucrose | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Cellulose | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Corn starch | 65.55 | 64.95 | 60.55 | 60.05 | 60.75 | 65.55 | 64.95 | 60.55 | 60.05 |
| Tert-butylhydroquinone | 0.0008 | 0.0008 | 0.0008 | 0.0008 | 0.0008 | 0.0008 | 0.0008 | 0.0008 | 0.0008 |
| Total (g or %) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Alonso, J.J.; Guillén-Mancina, E.; Calderón-Montaño, J.M.; Jiménez-González, V.; Díaz-Ortega, P.; Burgos-Morón, E.; López-Lázaro, M. Artificial Diets with Altered Levels of Sulfur Amino Acids Induce Anticancer Activity in Mice with Metastatic Colon Cancer, Ovarian Cancer and Renal Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 4587. https://doi.org/10.3390/ijms24054587
Jiménez-Alonso JJ, Guillén-Mancina E, Calderón-Montaño JM, Jiménez-González V, Díaz-Ortega P, Burgos-Morón E, López-Lázaro M. Artificial Diets with Altered Levels of Sulfur Amino Acids Induce Anticancer Activity in Mice with Metastatic Colon Cancer, Ovarian Cancer and Renal Cell Carcinoma. International Journal of Molecular Sciences. 2023; 24(5):4587. https://doi.org/10.3390/ijms24054587
Chicago/Turabian StyleJiménez-Alonso, Julio José, Emilio Guillén-Mancina, José Manuel Calderón-Montaño, Víctor Jiménez-González, Patricia Díaz-Ortega, Estefanía Burgos-Morón, and Miguel López-Lázaro. 2023. "Artificial Diets with Altered Levels of Sulfur Amino Acids Induce Anticancer Activity in Mice with Metastatic Colon Cancer, Ovarian Cancer and Renal Cell Carcinoma" International Journal of Molecular Sciences 24, no. 5: 4587. https://doi.org/10.3390/ijms24054587