Anti-DNA-IgM Favors the Detection of NET-Associated Extracellular DNA
Abstract
:1. Introduction
2. Results
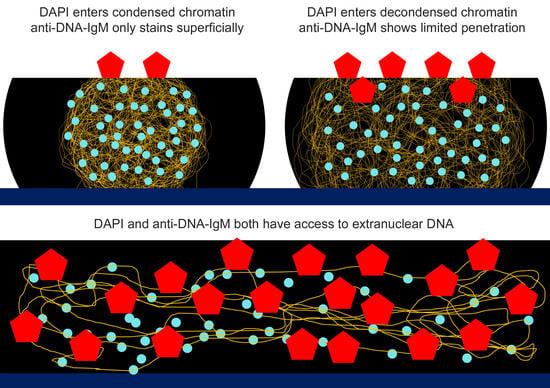
2.1. Anti-DNA-IgM Is Superior in Detecting Decondensed DNA In Vivo
2.2. Anti-DNA-IgM Is Suitable for High Sensitive Detection of NETs and ecDNA
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Tissue Collection
4.3. Isolation of Neutrophils
4.4. Staining of Tissue Sections
4.5. NET Formation In Vitro
4.6. Microscopy
4.7. Morphometry
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onufriev, A.V.; Schiessel, H. The nucleosome: From structure to function through physics. Curr. Opin. Struct. Biol. 2019, 56, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Domínguez, I.J.; Manzo-Merino, J.; Taja-Chayeb, L.; Dueñas-González, A.; Pérez-Cárdenas, E.; Trejo-Becerril, C. The role of extracellular DNA (exDNA) in cellular processes. Cancer Biol. Ther. 2021, 22, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D. Mechanisms of Chromatin Remodeling and Repurposing During Extracellular Translocation. Adv. Protein Chem. Struct. Biol. 2017, 106, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Mobarrez, F.; Fuzzi, E.; Gunnarsson, I.; Larsson, A.; Eketjäll, S.; Pisetsky, D.S.; Svenungsson, E. Microparticles in the blood of patients with SLE: Size, content of mitochondria and role in circulating immune complexes. J. Autoimmun. 2019, 102, 142–149. [Google Scholar] [CrossRef]
- Mobarrez, F.; Vikerfors, A.; Gustafsson, J.T.; Gunnarsson, I.; Zickert, A.; Larsson, A.; Pisetsky, D.S.; Wallén, H.; Svenungsson, E. Microparticles in the blood of patients with systemic lupus erythematosus (SLE): Phenotypic characterization and clinical associations. Sci. Rep. 2016, 6, 36025. [Google Scholar] [CrossRef] [Green Version]
- Beyer, C.; Pisetsky, D.S. Modeling nuclear molecule release during in vitro cell death. Autoimmunity 2013, 46, 298–301. [Google Scholar] [CrossRef]
- Mázló, A.; Jenei, V.; Burai, S.; Molnár, T.; Bácsi, A.; Koncz, G. Types of necroinflammation, the effect of cell death modalities on sterile inflammation. Cell Death Dis. 2022, 13, 423. [Google Scholar] [CrossRef]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release mechanisms of major DAMPs. Apoptosis 2021, 26, 152–162. [Google Scholar] [CrossRef]
- Pisetsky, D.S. The central role of nucleic acids in the pathogenesis of systemic lupus erythematosus. F1000Research 2019, 8, 368. [Google Scholar] [CrossRef] [Green Version]
- Okude, H.; Ori, D.; Kawai, T. Signaling Through Nucleic Acid Sensors and Their Roles in Inflammatory Diseases. Front. Immunol. 2021, 11, 625833. [Google Scholar] [CrossRef]
- Boeltz, S.; Amini, P.; Anders, H.-J.; Andrade, F.; Bilyy, R.; Chatfield, S.; Cichon, I.; Clancy, D.M.; Desai, J.; Dumych, T.; et al. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019, 26, 395–408. [Google Scholar] [CrossRef] [Green Version]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [Green Version]
- Leshner, M.; Wang, S.; Lewis, C.; Zheng, H.; Chen, X.A.; Santy, L.; Wang, Y. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 2012, 3, 307. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, M.; Stadler, S.C.; Correll, S.; Li, P.; Wang, D.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Lyu, X.; Liao, J.; Werth, V.P.; Liu, M.-L. Rho Kinase regulates neutrophil NET formation that is involved in UVB-induced skin inflammation. Theranostics 2022, 12, 2133–2149. [Google Scholar] [CrossRef]
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. A Myeloperoxidase-Containing Complex Regulates Neutrophil Elastase Release and Actin Dynamics during NETosis. Cell Rep. 2014, 8, 883–896. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Kronbichler, A.; Park, D.D.-Y.; Park, Y.; Moon, H.; Kim, H.; Choi, J.H.; Choi, Y.; Shim, S.; Lyu, I.S.; et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun. Rev. 2017, 16, 1160–1173. [Google Scholar] [CrossRef]
- Masuda, S.; Nakazawa, D.; Shida, H.; Miyoshi, A.; Kusunoki, Y.; Tomaru, U.; Ishizu, A. NETosis markers: Quest for specific, objective, and quantitative markers. Clin. Chim. Acta 2016, 459, 89–93. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Krumbholz, M.; Schönermarck, U.; Back, W.; Gross, W.L.; Werb, Z.; Gröne, H.-J.; Brinkmann, V.; Jenne, D.E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009, 15, 623–625. [Google Scholar] [CrossRef]
- Brinkmann, V.; Abu Abed, U.; Goosmann, C.; Zychlinsky, A. Immunodetection of NETs in Paraffin-Embedded Tissue. Front. Immunol. 2016, 7, 513. [Google Scholar] [CrossRef] [Green Version]
- Rebernick, R.; Fahmy, L.; Glover, C.; Bawadekar, M.; Shim, D.; Holmes, C.L.; Rademacher, N.; Potluri, H.; Bartels, C.M.; Shelef, M.A. DNA Area and NETosis Analysis (DANA): A High-Throughput Method to Quan tify Neutrophil Extracellular Traps in Fluorescent Microscope Images. Biol. Proced. Online 2018, 20, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagstaff, K.; Jans, D. Nucleocytoplasmic transport of DNA: Enhancing non-viral gene transfer. Biochem. J. 2007, 406, 185–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poon, I.; Jans, D. Regulation of Nuclear Transport: Central Role in Development and Transformation? Traffic 2005, 6, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Meng, Q.; Liu, K. Peptide-based gene delivery vectors. J. Mater. Chem. B 2019, 7, 1824–1841. [Google Scholar] [CrossRef]
- Karoui, H.; Patwal, P.S.; Kumar, B.V.V.S.P.; Martin, N. Chemical Communication in Artificial Cells: Basic Concepts, Design and Challenges. Front. Mol. Biosci. 2022, 9, 880525. [Google Scholar] [CrossRef]
- Lukacs, G.L.; Haggie, P.; Seksek, O.; Lechardeur, D.; Freedman, N.; Verkman, A.S. Size-dependent DNA Mobility in Cytoplasm and Nucleus. J. Biol. Chem. 2000, 275, 1625–1629. [Google Scholar] [CrossRef] [Green Version]
- Biancardi, A.; Biver, T.; Secco, F.; Mennucci, B. An investigation of the photophysical properties of minor groove bound and intercalated DAPI through quantum-mechanical and spectroscopic tools. Phys. Chem. Chem. Phys. 2013, 15, 4596–4603. [Google Scholar] [CrossRef]
- Barcellona, M.L.; Gratton, E. The fluorescence properties of a DNA probe. Eur. Biophys. J. 1990, 17, 315–323. [Google Scholar] [CrossRef]
- Wilson, W.D.; Tanious, F.A.; Barton, H.J.; Jones, R.L.; Strekowski, L.; Boykin, D.W. Binding of 4’,6-diamidino-2-phenylindole (DAPI) to GC and mixed sequences in DNA: Intercalation of a classical groove-binding molecule. J. Am. Chem. Soc. 1989, 111, 5008–5010. [Google Scholar] [CrossRef]
- Trotta, E.; D’Ambrosio, E.; Ravagnan, G.; Paci, M. Simultaneous and Different Binding Mechanisms of 4′,6-Diamidino-2-phenylindole to DNA Hexamer (d(CGATCG))2: A 1H NMR STUDY. J. Biol. Chem. 1996, 271, 27608–27614. [Google Scholar] [CrossRef] [Green Version]
- Mora, A.K.; Khan, S.; Patro, B.S.; Nath, S. Is DAPI assay of cellular nucleic acid reliable in the presence of protein aggregates? Chem. Commun. 2020, 56, 13844–13847. [Google Scholar] [CrossRef]
- Jiang, N.; Reich, C.F.; Monestier, M.; Pisetsky, D.S. The expression of plasma nucleosomes in mice undergoing in vivo apoptosis. Clin. Immunol. 2003, 106, 139–147. [Google Scholar] [CrossRef]
- Jiang, N.; Reich, C.F.; Pisetsky, D.S. Role of macrophages in the generation of circulating blood nucleosomes from dead and dying cells. Blood 2003, 102, 2243–2250. [Google Scholar] [CrossRef] [Green Version]
- Lo, Y.M.D.; Han, D.S.C.; Jiang, P.; Chiu, R.W.K. Epigenetics, fragmentomics, and topology of cell-free DNA in liquid biopsies. Science 2021, 372, eaaw3616. [Google Scholar] [CrossRef]
- Gagnon, P.; Hensel, F.; Lee, S.; Zaidi, S. Chromatographic behavior of IgM:DNA complexes. J. Chromatogr. A 2011, 1218, 2405–2412. [Google Scholar] [CrossRef]
- Luzwick, J.W.; Dombi, E.; Boisvert, R.A.; Roy, S.; Park, S.; Kunnimalaiyaan, S.; Goffart, S.; Schindler, D.; Schlacher, K. MRE11-dependent instability in mitochondrial DNA fork protection activates a cGAS immune signaling pathway. Sci. Adv. 2021, 7, eabf9441. [Google Scholar] [CrossRef]
- Simon, B.; Bolumar, D.; Amadoz, A.; Jimenez-Almazán, J.; Valbuena, D.; Vilella, F.; Moreno, I. Identification and Characterization of Extracellular Vesicles and Its DNA Cargo Secreted During Murine Embryo Development. Genes 2020, 11, 203. [Google Scholar] [CrossRef] [Green Version]
- Akhouri, R.R.; Goel, S.; Furusho, H.; Skoglund, U.; Wahlgren, M. Architecture of Human IgM in Complex with P. falciparum Erythrocyte Membrane Protein 1. Cell Rep. 2016, 14, 723–736. [Google Scholar] [CrossRef] [Green Version]
- Fiorelli, R.; Sidhu, G.S.; Cebrián-Silla, A.; Melendez, E.L.; Mehta, S.; Garcia-Verdugo, J.M.; Sanai, N. Enhanced tissue penetration of antibodies through pressurized immunohistochemistry. bioRxiv 2020. [Google Scholar] [CrossRef]
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.H.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.Y.; Surette, M.G.; Sugai, M.; et al. A Novel Mechanism of Rapid Nuclear Neutrophil Extracellular Trap Formation in Response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stehr, A.M.; Wang, G.; Demmler, R.; Stemmler, M.P.; Krug, J.; Tripal, P.; Schmid, B.; Geppert, C.I.; Hartmann, A.; Muñoz, L.E.; et al. Neutrophil extracellular traps drive epithelial–mesenchymal transition of human colon cancer. J. Pathol. 2022, 256, 455–467. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Stehr, A.M.; Singh, J.; Zlatar, L.; Hartmann, A.; Evert, K.; Naschberger, E.; von Stillfried, S.; Boor, P.; Muñoz, L.E.; et al. Anti-DNA-IgM Favors the Detection of NET-Associated Extracellular DNA. Int. J. Mol. Sci. 2023, 24, 4101. https://doi.org/10.3390/ijms24044101
Wang H, Stehr AM, Singh J, Zlatar L, Hartmann A, Evert K, Naschberger E, von Stillfried S, Boor P, Muñoz LE, et al. Anti-DNA-IgM Favors the Detection of NET-Associated Extracellular DNA. International Journal of Molecular Sciences. 2023; 24(4):4101. https://doi.org/10.3390/ijms24044101
Chicago/Turabian StyleWang, Han, Antonia Margarethe Stehr, Jeeshan Singh, Leticija Zlatar, Arndt Hartmann, Katja Evert, Elisabeth Naschberger, Saskia von Stillfried, Peter Boor, Luis E. Muñoz, and et al. 2023. "Anti-DNA-IgM Favors the Detection of NET-Associated Extracellular DNA" International Journal of Molecular Sciences 24, no. 4: 4101. https://doi.org/10.3390/ijms24044101