A Three-Dimensional Xeno-Free Culture Condition for Wharton’s Jelly-Mesenchymal Stem Cells: The Pros and Cons
Abstract
:1. Introduction
2. Results
2.1. Human Platelet Lysate as a Reliable FBS Substitute for Xeno-Free Culture
2.1.1. Human Platelet Lysate Culture Media Alters WJMSCs Morphology and Growth in the 2D Culture Setting
2.1.2. MSCs Cultured in LG-HPL Showed Characteristics in Accordance to the International Society for Cellular Therapy’s Guidelines
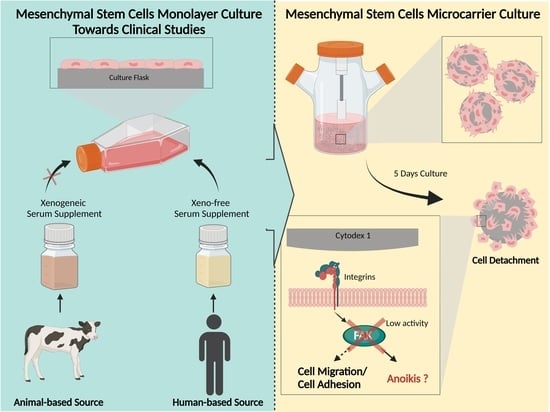
2.2. WJMSCs Microcarrier Culture in Xeno-Free Culture Condition
2.2.1. MSCs Microcarrier Culture in LG-HPL Media Showing Stagnated Proliferation
2.2.2. MSC Microcarrier Cultures Retained Their Characteristic as MSCs after Being Cultured on Microcarriers
2.2.3. MSCs Microcarrier Culture Maintaining High Inhibitory Activity on TNF-α and IL-1 Secretion by PBMCs
3. Discussion
4. Materials and Methods
4.1. WJMSC Isolations and Culture
4.2. Calculation of Population Doubling Time (PDT)
4.3. Analysis of Cell Viability
4.4. Microcarrier Preparation
4.5. WJMSCs Microcarrier Culture
4.6. WJMSCs Immunophenotypic Analysis and Differentiation Analysis
4.7. Immunosuppression Assay
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abomaray, F.M.; Al Jumah, M.A.; Kalionis, B.; AlAskar, A.S.; Al Harthy, S.; Jawdat, D.; Al Khaldi, A.; Alkushi, A.; Knawy, B.A.; Abumaree, M.H. Human Chorionic Villous Mesenchymal Stem Cells Modify the Functions of Human Dendritic Cells, and Induce an Anti-Inflammatory Phenotype in CD1+ Dendritic Cells. Stem Cell Rev. Rep. 2015, 11, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Armstrong, M.A.; Li, G. Mesenchymal Stem Cells in Immunoregulation. Immunol. Cell Biol. 2006, 84, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Keating, A. Mesenchymal Stromal Cells. In Current Opinion in Hematology; Lippincott Williams and Wilkins Ltd.: Philadelphia, PA, USA, 2006; pp. 419–425. [Google Scholar] [CrossRef]
- Liau, L.L.; Ruszymah, B.H.I.; Ng, M.H.; Law, J.X. Characteristics and Clinical Applications of Wharton’s Jelly-Derived Mesenchymal Stromal Cells. Curr. Res. Transl. Med. 2020, 68, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.S.; Yazid, M.D.; Sainik, N.Q.A.V.; Razali, R.A.; Bin Saim, A.; Idrus, R.B.H. Osteogenic Induction of Wharton’s Jelly-Derived Mesenchymal Stem Cell for Bone Regeneration: A Systematic Review. Stem Cells Int. 2018, 2018, 2406462. [Google Scholar] [CrossRef]
- Leow, S.N.; Luu, C.D.; Nizam, M.H.H.; Mok, P.L.; Ruhaslizan, R.; Wong, H.S.; Halim, W.H.W.A.; Ng, M.H.; Ruszymah, B.H.I.; Chowdhury, S.R.; et al. Safety and Efficacy of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells Therapy for Retinal Degeneration. PLoS ONE 2015, 10, e0128973. [Google Scholar] [CrossRef]
- Moll, G.; Drzeniek, N.; Kamhieh-Milz, J.; Geissler, S.; Volk, H.D.; Reinke, P. MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy. Front. Immunol. 2020, 11, 1091. [Google Scholar] [CrossRef]
- Rossello-Gelabert, M.; Gonzalez-Pujana, A.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Clinical Progress in MSC-Based Therapies for the Management of Severe COVID-19. Cytokine Growth Factor Rev. 2022, 68, 25–36. [Google Scholar] [CrossRef]
- Rezakhani, L.; Kelishadrokhi, A.F.; Soleimanizadeh, A.; Rahmati, S. Mesenchymal Stem Cell (MSC)-Derived Exosomes as a Cell-Free Therapy for Patients Infected with COVID-19: Real Opportunities and Range of Promises. Chem. Phys. Lipids 2021, 234, 105009. [Google Scholar] [CrossRef]
- Kavianpour, M.; Saleh, M.; Verdi, J. The Role of Mesenchymal Stromal Cells in Immune Modulation of COVID-19: Focus on Cytokine Storm. Stem Cell Res. Ther. 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Cayetano, S.M.; Alvarez, R.A.; Kouroupis, D.; Gil, A.A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical Cord Mesenchymal Stem Cells for COVID-19 Acute Respiratory Distress Syndrome: A Double-Blind, Phase 1/2a, Randomized Controlled Trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef]
- Song, N.; Wakimoto, H.; Rossignoli, F.; Bhere, D.; Ciccocioppo, R.; Chen, K.S.; Khalsa, J.K.; Mastrolia, I.; Samarelli, A.V.; Dominici, M.; et al. Mesenchymal Stem Cell Immunomodulation: In Pursuit of Controlling COVID-19 Related Cytokine Storm. Stem Cells 2021, 39, 707–722. [Google Scholar] [CrossRef]
- Moradinasab, S.; Pourbagheri-Sigaroodi, A.; Zafari, P.; Ghaffari, S.H.; Bashash, D. Mesenchymal Stromal/Stem Cells (MSCs) and MSC-Derived Extracellular Vesicles in COVID-19-Induced ARDS: Mechanisms of Action, Research Progress, Challenges, and Opportunities. Int. Immunopharmacol. 2021, 97, 107694. [Google Scholar] [CrossRef]
- Alsalem, M.A.; Albahri, O.S.; Zaidan, A.A.; Al-Obaidi, J.R.; Alnoor, A.; Alamoodi, A.H.; Albahri, A.S.; Zaidan, B.B.; Jumaah, F.M. Rescuing Emergency Cases of COVID-19 Patients: An Intelligent Real-Time MSC Transfusion Framework Based on Multicriteria Decision-Making Methods. Appl. Intell. 2022, 52, 9676–9700. [Google Scholar] [CrossRef]
- YekrangSafakar, A.; Acun, A.; Choi, J.-W.; Song, E.; Zorlutuna, P.; Park, K. Hollow Microcarriers for Large-Scale Expansion of Anchorage-Dependent Cells in a Stirred Bioreactor. Biotechnol. Bioeng. 2018, 115, 1717–1728. [Google Scholar] [CrossRef]
- Kim, S.N.; Lee, C.J.; Nam, J.H.; Choi, B.; Chung, E.; Song, S.U. The Effects of Human Bone Marrow-Derived Mesenchymal Stem Cell Conditioned Media Produced with Fetal Bovine Serum or Human Platelet Lysate on Skin Rejuvenation Characteristics. Int. J. Stem Cells 2020, 14, 94–102. [Google Scholar] [CrossRef]
- Kandoi, S.; Kumar, L.P.; Patra, B.; Vidyasekar, P.; Sivanesan, D.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. Evaluation of Platelet Lysate as a Substitute for FBS in Explant and Enzymatic Isolation Methods of Human Umbilical Cord MSCs. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Burnouf, T.; Strunk, D.; Koh, M.B.C.; Schallmoser, K. Human Platelet Lysate: Replacing Fetal Bovine Serum as a Gold Standard for Human Cell Propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef]
- Bieback, K.; Red, G. Platelet Lysate as Replacement for Fetal Bovine Serum in Mesenchymal Stromal Cell Cultures. Transfus. Med. Hemother. 2013, 40, 326–335. [Google Scholar] [CrossRef]
- van der Valk, J.; Brunner, D.; De Smet, K.; Svenningsen, Å.F.; Honegger, P.; Knudsen, L.E.; Lindl, T.; Noraberg, J.; Price, A.; Scarino, M.L.; et al. Optimization of Chemically Defined Cell Culture Media--Replacing Fetal Bovine Serum in Mammalian in Vitro Methods. Toxicol. In Vitro 2010, 24, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Spees, J.L.; Gregory, C.A.; Singh, H.; Tucker, H.A.; Peister, A.; Lynch, P.J.; Hsu, S.C.; Smith, J.; Prockop, D.J. Internalized Antigens Must Be Removed to Prepare Hypoimmunogenic Mesenchymal Stem Cells for Cell and Gene Therapy. Mol. Ther. 2004, 9, 747–756. [Google Scholar] [CrossRef]
- Gstraunthaler, G.; Lindl, T.; Van Der Valk, J. A Severe Case of Fraudulent Blending of Fetal Bovine Serum Strengthens the Case for Serum-Free Cell and Tissue Culture Applications. ATLA Altern. Lab. Anim. 2014, 42, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Price, P.J.; Gregory, E.A. Relationship between in Vitro Growth Promotion and Biophysical and Biochemical Properties of the Serum Supplement. In Vitro 1982, 18, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Vojgani, Y.; Shirazi, A.; Zarei, S.; Yeganeh, O.; Jeddi-Tehrani, M. Comparison of Efficacies of Fetal Bovine Sera from Different Suppliers in Cell Culture Experiments. Comp. Clin. Path. 2017, 27, 519–527. [Google Scholar] [CrossRef]
- de Peppo, G.M. GMP-Compatible, Xeno-Free Culture of Human Induced Mesenchymal Stem Cells. In Methods in Molecular Biology; Humana Press Inc.: Louisville, KY, USA, 2021; Volume 2286, pp. 121–129. [Google Scholar] [CrossRef]
- Comella, K.; Parlo, M.; Daly, R.; Depasquale, V.; Edgerton, E.; Mallory, P.; Schmidt, R.; Drake, W.P. Safety Analysis of Autologous Stem Cell Therapy in a Variety of Degenerative Diseases and Injuries Using the Stromal Vascular Fraction. J. Clin. Med. Res. 2017, 9, 935–942. [Google Scholar] [CrossRef]
- Li, C.Y.; Wu, X.Y.; Tong, J.B.; Yang, X.X.; Zhao, J.L.; Zheng, Q.F.; Zhao, G.B.; Ma, Z.J. Comparative Analysis of Human Mesenchymal Stem Cells from Bone Marrow and Adipose Tissue under Xeno-Free Conditions for Cell Therapy. Stem Cell Res. Ther. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Russell, A.L.; Lefavor, R.C.; Zubair, A.C. Characterization and Cost–Benefit Analysis of Automated Bioreactor-Expanded Mesenchymal Stem Cells for Clinical Applications. Transfusion 2018, 58, 2374–2382. [Google Scholar] [CrossRef]
- Lawson, T.; Kehoe, D.E.; Schnitzler, A.C.; Rapiejko, P.J.; Der, K.A.; Philbrick, K.; Punreddy, S.; Rigby, S.; Smith, R.; Feng, Q.; et al. Process Development for Expansion of Human Mesenchymal Stromal Cells in a 50 L Single-Use Stirred Tank Bioreactor. Biochem. Eng. J. 2017, 120, 49–62. [Google Scholar] [CrossRef]
- Hassan, M.N.F.B.; Yap, Y.; Tang, Y.L.; Ng, M.H.; Jia, X.L. Expired Platelet Concentrate as a Source of Human Platelet Lysate for Xenogeneic-Free Culture of Human Dermal Fibroblasts (Platelet Pekat Tamat Tempoh Sebagai Sumber Platelet Lisat Manusia Untuk Pengkulturan Sel Fibroblas Kulit Manusia Secara Bebas Xenogenik). Sains Malays. 2021, 50, 2355–2365. [Google Scholar] [CrossRef]
- Jonsdottir-Buch, S.M.; Lieder, R.; Sigurjonsson, O.E. Platelet Lysates Produced from Expired Platelet Concentrates Support Growth and Osteogenic Differentiation of Mesenchymal Stem Cells. PLoS ONE 2013, 8, e68984. [Google Scholar] [CrossRef]
- Yang, P.; Chen, X.; Kaushal, S.; Reece, E.A.; Yang, P. High Glucose Suppresses Embryonic Stem Cell Differentiation into Cardiomyocytes: High Glucose Inhibits ES Cell Cardiogenesis. Stem Cell Res. Ther. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Nan, L.P.; Wang, F.; Zhou, S.F.; Wang, J.C.; Feng, X.M.; Zhang, L. The Effect of High Glucose on the Biological Characteristics of Nucleus Pulposus-Derived Mesenchymal Stem Cells. Cell Biochem. Funct. 2020, 38, 130–140. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Muñoz, B.F.; Lopez-Navas, L.; Bermejo, M.G.; Romero, I.M.L.; Aguilera, M.Á.M.; Cuerva, R.C.; Arribas, B.A.; Nogueras, S.; Sánchez, G.C.; González, M.S. A proprietary gmp human platelet lysate for the expansion of dermal fibroblasts for clinical applications. Platelets 2021, 33, 98–109. [Google Scholar] [CrossRef]
- Heathman, T.R.J.; Stolzing, A.; Fabian, C.; Rafiq, Q.A.; Coopman, K.; Nienow, A.W.; Kara, B.; Hewitt, C.J. Scalability and Process Transfer of Mesenchymal Stromal Cell Production from Monolayer to Microcarrier Culture Using Human Platelet Lysate. Cytotherapy 2016, 18, 523–535. [Google Scholar] [CrossRef]
- Czapla, J.; Matuszczak, S.; Kulik, K.; Wiśniewska, E.; Pilny, E.; Jarosz-Biej, M.; Smolarczyk, R.; Sirek, T.; Zembala, M.O.; Zembala, M.; et al. The Effect of Culture Media on Large-Scale Expansion and Characteristic of Adipose Tissue-Derived Mesenchymal Stromal Cells. Stem Cell Res. Ther. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Hemeda, H.; Giebel, B.; Wagner, W. Evaluation of Human Platelet Lysate versus Fetal Bovine Serum for Culture of Mesenchymal Stromal Cells. Cytotherapy 2014, 16, 170–180. [Google Scholar] [CrossRef]
- Heathman, T.R.J.; Nienow, A.W.; Rafiq, Q.A.; Coopman, K.; Kara, B.; Hewitt, C.J. Agitation and Aeration of Stirred-Bioreactors for the Microcarrier Culture of Human Mesenchymal Stem Cells and Potential Implications for Large-Scale Bioprocess Development. Biochem. Eng. J. 2018, 136, 9–17. [Google Scholar] [CrossRef]
- Shanskii, Y.D.; Sergeeva, N.S.; Sviridova, I.K.; Kirakozov, M.S.; Kirsanova, V.A.; Akhmedova, S.A.; Antokhin, A.I.; Chissov, V.I. Human Platelet Lysate as a Promising Growth-Stimulating Additive for Culturing of Stem Cells and Other Cell Types. Bull. Exp. Biol. Med. 2013, 156, 146–151. [Google Scholar] [CrossRef]
- Pasztorek, M.; Rossmanith, E.; Mayr, C.; Hauser, F.; Jacak, J.; Ebner, A.; Weber, V.; Fischer, M.B. Influence of Platelet Lysate on 2D and 3D Amniotic Mesenchymal Stem Cell Cultures. Front. Bioeng. Biotechnol. 2019, 7, 338. [Google Scholar] [CrossRef]
- Hewitt, C.J.; Lee, K.; Nienow, A.W.; Thomas, R.J.; Smith, M.; Thomas, C.R. Expansion of Human Mesenchymal Stem Cells on Microcarriers. Biotechnol. Lett. 2011, 33, 2325–2335. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, K.; Yang, Y.; Deng, D.; Lyu, C.; Xu, H.; Liu, W.; Du, Y. Dispersible and Dissolvable Porous Microcarrier Tablets Enable Efficient Large-Scale Human Mesenchymal Stem Cell Expansion. Tissue Eng.—Part C Methods 2020, 26, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Tao, X.; Liu, K.; Lin, J.; Shi, Y.; Park, K.; Chen, H.Y.; Lin, C.P.; Chang, J.; Wong, R.C.; et al. Human Platelet Lysate (HPL) Alters the Lineage Commitment and Paracrine Functions of Human Mesenchymal Stem Cells via Mitochondrial Metabolism. Appl. Mater. Today 2022, 26, 101264. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, Z.; Webster, K.A.; Chen, J.; Hu, H.; Zhou, Y.; Zhao, J.; Wang, L.; Wang, Y.; Zhong, Z.; et al. Enhanced Cardioprotection by Human Endometrium Mesenchymal Stem Cells Driven by Exosomal MicroRNA-21. Stem Cells Transl. Med. 2017, 6, 209–222. [Google Scholar] [CrossRef]
- Samakova, A.; Gazova, A.; Sabova, N.; Valaskova, S.; Jurikova, M.; Kyselovic, J. The Pi3k/Akt Pathway Is Associated with Angiogenesis, Oxidative Stress and Survival of Mesenchymal Stem Cells in Pathophysiologic Condition in Ischemia. Physiol. Res. 2019, 68 (Suppl. S2), S131–S138. [Google Scholar] [CrossRef]
- Li, W.; Ma, N.; Ong, L.-L.; Nesselmann, C.; Klopsch, C.; Ladilov, Y.; Furlani, D.; Piechaczek, C.; Moebius, J.M.; Lützow, K.; et al. Bcl-2 Engineered MSCs Inhibited Apoptosis and Improved Heart Function. Stem Cells 2007, 25, 2118–2127. [Google Scholar] [CrossRef]
- Wang, Z.J.; Zhang, F.M.; Wang, L.S.; Yao, Y.W.; Zhao, Q.; Gao, X. Lipopolysaccharides Can Protect Mesenchymal Stem Cells (MSCs) from Oxidative Stress-Induced Apoptosis and Enhance Proliferation of MSCs via Toll-like Receptor(TLR)-4 and PI3K/Akt. Cell Biol. Int. 2009, 33, 665–674. [Google Scholar] [CrossRef]
- Jang, M.W.; Yun, S.P.; Park, J.H.; Ryu, J.M.; Lee, J.H.; Han, H.J. Cooperation of Epac1/Rap1/Akt and PKA in Prostaglandin E(2) -Induced Proliferation of Human Umbilical Cord Blood Derived Mesenchymal Stem Cells: Involvement of c-Myc and VEGF Expression. J. Cell. Physiol. 2012, 227, 3756–3767. [Google Scholar] [CrossRef]
- Hall, J.E.; Fu, W.; Schaller, M.D. Focal Adhesion Kinase: Exploring FAK Structure to Gain Insight into Function. Int. Rev. Cell Mol. Biol. 2011, 288, 185–225. [Google Scholar] [CrossRef]
- Blaukat, A. Focal Adhesion Kinase. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–10. [Google Scholar] [CrossRef]
- Hanks, S.K. FAK Family. In Encyclopedia of Biological Chemistry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 265–268. [Google Scholar] [CrossRef]
- Rosen, G.D.; Dube, D.S. ADHESION, CELL–MATRIX | Focal Contacts and Signaling. In Encyclopedia of Respiratory Medicine; Academic Press: Cambridge, MA, USA, 2006; pp. 41–47. [Google Scholar] [CrossRef]
- Song, H.; Chang, W.; Lim, S.; Seo, H.-S.; Shim, C.Y.; Park, S.; Yoo, K.-J.; Kim, B.-S.; Min, B.-H.; Lee, H.; et al. Tissue Transglutaminase Is Essential for Integrin-Mediated Survival of Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells 2007, 25, 1431–1438. [Google Scholar] [CrossRef]
- Song, H.; Cha, M.J.; Song, B.W.; Kim, I.K.; Chang, W.; Lim, S.; Choi, E.J.; Ham, O.; Lee, S.Y.; Chung, N.; et al. Reactive Oxygen Species Inhibit Adhesion of Mesenchymal Stem Cells Implanted into Ischemic Myocardium via Interference of Focal Adhesion Complex. Stem Cells 2010, 28, 555–563. [Google Scholar] [CrossRef]
- Ben-Mahdi, M.H.; Dang, P.M.C.; Gougerot-Pocidalo, M.A.; O’Dowd, Y.; El-Benna, J.; Pasquier, C. Xanthine Oxidase-Derived ROS Display a Biphasic Effect on Endothelial Cells Adhesion and FAK Phosphorylation. Oxid. Med. Cell. Longev. 2016, 2016, 9346242. [Google Scholar] [CrossRef]
- Xu, C.S.; Wang, Z.F.; Huang, X.D.; Dai, L.M.; Cao, C.J.; Li, Z.Q. Involvement of ROS-Alpha v Beta 3 Integrin-FAK/Pyk2 in the Inhibitory Effect of Melatonin on U251 Glioma Cell Migration and Invasion under Hypoxia. J. Transl. Med. 2015, 13, 1–11. [Google Scholar] [CrossRef]
- Frisch, S.M. Anoikis. Methods Enzymol. 2000, 322, 472–479. [Google Scholar] [CrossRef]
- Maillard, E. Plasma Scaffolds for Islet Transplantation. In Transplantation, Bioengineering, and Regeneration of the Endocrine Pancreas; Academic Press: Cambridge, MA, USA, 2020; Volume 2, pp. 257–268. [Google Scholar] [CrossRef]
- Bjerknes, M.; Cheng, H. Intestinal Epithelial Stem Cells and Progenitors. Methods Enzymol. 2006, 419, 337–383. [Google Scholar] [CrossRef]
- Rankin, E.B.; Erler, J.; Giaccia, A.J. The Cellular Microenvironment and Metastases. In Abeloff’s Clinical Oncology, 5th ed.; Churchill Livingstone: Lodon, UK, 2014; pp. 40–51.e4. [Google Scholar] [CrossRef]
- Malagobadan, S.; Nagoor, N.H. Anoikis. In Encyclopedia of Cancer, 3rd ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 75–84. [Google Scholar] [CrossRef]
- Ge, J.; Huang, Y.; Lv, M.W.; Zhang, C.; Talukder, M.; Li, J.Y.; Li, J.L. Cadmium Induced Fak -Mediated Anoikis Activation in Kidney via Nuclear Receptors (AHR/CAR/PXR)-Mediated Xenobiotic Detoxification Pathway. J. Inorg. Biochem. 2022, 227, 111682. [Google Scholar] [CrossRef]
- Li, K.; Zhao, G.; Ao, J.; Gong, D.; Zhang, J.; Chen, Y.; Li, J.; Huang, L.; Xiang, R.; Hu, J.; et al. ZNF32 Induces Anoikis Resistance through Maintaining Redox Homeostasis and Activating Src/FAK Signaling in Hepatocellular Carcinoma. Cancer Lett. 2019, 442, 271–278. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Q.; Wu, S.; Zhang, H.; Yu, W. Mst1 Inhibition as a Cellular Mediator: Prevention of Anoikis in MBMSCs through Activating ITGα5β1/FAK Signaling Pathway. 2021. Available online: https://doi.org/10.21203/rs.3.rs-977461/v1 (accessed on 21 January 2023).
- Miao, Y.; Lu, J.; Yin, J.; Zhou, C.; Guo, Y.; Zhou, S. Yb3+-Containing Chitosan Hydrogels Induce B-16 Melanoma Cell Anoikis via a Fak-Dependent Pathway. Nanotechnol. Rev. 2019, 8, 645–660. [Google Scholar] [CrossRef]
- Mo, C.F.; Li, J.; Yang, S.X.; Guo, H.J.; Liu, Y.; Luo, X.Y.; Wang, Y.T.; Li, M.H.; Li, J.Y.; Zou, Q. IQGAP1 Promotes Anoikis Resistance and Metastasis through Rac1-Dependent ROS Accumulation and Activation of Src/FAK Signalling in Hepatocellular Carcinoma. Br. J. Cancer 2020, 123, 1154–1163. [Google Scholar] [CrossRef]
- Yang, C.; Denggao, H.; Yuanhui, G.; Shunlan, W.; Hui, C.; Linlin, Z.; Haowei, H.; Siqin, L.; Jingchuan, X.; Yingai, Z.; et al. Low-Intensity Pulsed Ultrasound Promotes the Proliferation and Adhesion of Human Adipose-Derived Mesenchymal Stem Cells. Chin. J. Tissue Eng. Res. 2021, 25, 3949. [Google Scholar] [CrossRef]
- Koh, B.; Sulaiman, N.; Fauzi, M.B.; Law, J.X.; Ng, M.H.; Idrus, R.B.H.; Yazid, M.D. Three Dimensional Microcarrier System in Mesenchymal Stem Cell Culture: A Systematic Review. Cell Biosci. 2020, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, F.; Sarzi-Puttini, P. Tumor Necrosis Factor. In Brenner’s Encyclopedia of Genetics, 2nd ed.; 2013; pp. 229–231. [Google Scholar] [CrossRef]
- Wang, H.; Czura, C.J.; Tracey, K.J. Tumor Necrosis Factor. In The Cytokine Handbook, 4th ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 837–860. [Google Scholar] [CrossRef]
- Feldmann, J.; Prieur, A.M.; Quartier, P.; Berquin, P.; Certain, S.; Cortis, E.; Teillac-Harnel, D.; Fischer, A.; De Saint Basile, G. Chronic Infantile Neurological Cutaneous and Articular Syndrome Is Caused by Mutations in CIAS1, a Gene Highly Expressed in Polymorphonuclear Cells and Chondrocytes. Am. J. Hum. Genet. 2002, 71, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Goldbach-Mansky, R.; Dailey, N.J.; Canna, S.W.; Gelabert, A.; Jones, J.; Rubin, B.I.; Kim, H.J.; Brewer, C.; Zalewski, C.; Wiggs, E.; et al. Neonatal-Onset Multisystem Inflammatory Disease Responsive to Interleukin-1β Inhibition. N. Engl. J. Med. 2006, 355, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, J.; Rijkers, G.T.; Mandey, S.H.L.; Buurman, S.W.M.; Houten, S.M.; Wanders, R.J.A.; Waterham, H.R.; Kuis, W. Lack of Isoprenoid Products Raises Ex Vivo Interleukin-1β Secretion in Hyperimmunoglobulinemia D and Periodic Fever Syndrome. Arthritis Rheum. 2002, 46, 2794–2803. [Google Scholar] [CrossRef]
- Bizzi, E.; Trotta, L.; Pancrazi, M.; Nivuori, M.; Giosia, V.; Matteucci, L.; Montori, D.; Brucato, A. Autoimmune and Autoinflammatory Pericarditis: Definitions and New Treatments. Curr. Cardiol. Rep. 2021, 23, 1–11. [Google Scholar] [CrossRef]
- ter Haar, N.M.; Jansen, M.H.A.; Frenkel, J.F.; Vastert, S.J. How Autoinflammation May Turn into Autoimmune Inflammation: Insights from Monogenetic and Complex IL-1 Mediated Auto-Inflammatory Diseases. Clin. Immunol. 2020, 219, 108538. [Google Scholar] [CrossRef]
- Ruscitti, P.; Cipriani, P.; Liakouli, V.; Carubbi, F.; Berardicurti, O.; Di Benedetto, P.; Ciccia, F.; Guggino, G.; Alvaro, S.; Triolo, G.; et al. The Emerging Role of IL-1 Inhibition in Patients Affected by Rheumatoid Arthritis and Diabetes. Rev. Recent Clin. Trials 2018, 13, 210–214. [Google Scholar] [CrossRef]
- Wutthi-in, M.; Cheevadhanarak, S.; Yasom, S.; Kerdphoo, S.; Thiennimitr, P.; Phrommintikul, A.; Chattipakorn, N.; Kittichotirat, W.; Chattipakorn, S. Gut Microbiota Profiles of Treated Metabolic Syndrome Patients and Their Relationship with Metabolic Health. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic Syndrome Is an Inflammatory Disorder: A Conspiracy between Adipose Tissue and Phagocytes. Clin. Chim. Acta 2019, 496, 35–44. [Google Scholar] [CrossRef]
- Chan, A.M.L.; Ng, A.M.H.; Yunus, M.H.M.; Idrus, R.B.H.; Law, J.X.; Yazid, M.D.; Chin, K.Y.; Shamsuddin, S.A.; Lokanathan, Y. Recent Developments in Rodent Models of High-Fructose Diet-Induced Metabolic Syndrome: A Systematic Review. Nutrition 2021, 13, 2497. [Google Scholar] [CrossRef]
- Kritas, S.Κ.; Gallenga, C.E. Mast Cells Contribute to Coronavirus-Induced Inflammation: New Anti-Inflammatory Strategy SARS-CoV-2 Infection View Project Inflammatory Cytokines View Project. Artic. J. Biol. Regul. Homeost. Agents 2019, 34, 9–14. [Google Scholar] [CrossRef]
- Baker, K.J.; Houston, A.; Brint, E. IL-1 Family Members in Cancer; Two Sides to Every Story. Front. Immunol. 2019, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Gallenga, C.E. Induction of Pro-Inflammatory Cytokines (IL-1 and IL-6) and Lung Inflammation by COVID-19: Anti-Inflammatory Strategies. Artic. J. Biol. Regul. Homeost. Agents 2020, 34, 327–331. [Google Scholar] [CrossRef]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Barajon, I.; Garlanda, C. IL-1 and IL-1 Regulatory Pathways in Cancer Progression and Therapy. Immunol. Rev. 2018, 281, 57–61. [Google Scholar] [CrossRef]
- Wu, T.C.; Xu, K.; Martinek, J.; Young, R.R.; Banchereau, R.; George, J.; Turner, J.; Kim, K.I.; Zurawski, S.; Wang, X.; et al. IL1 Receptor Antagonist Controls Transcriptional Signature of Inflammation in Patients with Metastatic Breast Cancer. Cancer Res. 2018, 78, 5243–5258. [Google Scholar] [CrossRef]
- Moradian, N.; Gouravani, M.; Salehi, M.A.; Heidari, A.; Shafeghat, M.; Hamblin, M.R.; Rezaei, N. Cytokine Release Syndrome: Inhibition of pro-Inflammatory Cytokines as a Solution for Reducing COVID-19 Mortality. Eur. Cytokine Netw. 2020, 31, 81–93. [Google Scholar] [CrossRef]
- Robinson, P.C.; Liew, D.F.L.; Liew, J.W.; Monaco, C.; Richards, D.; Shivakumar, S.; Tanner, H.L.; Feldmann, M. The Potential for Repurposing Anti-TNF as a Therapy for the Treatment of COVID-19. Med 2020, 1, 90–102. [Google Scholar] [CrossRef]
- Duret, P.M.; Sebbag, E.; Mallick, A.; Gravier, S.; Spielmann, L.; Messer, L. Recovery from COVID-19 in a Patient with Spondyloarthritis Treated with TNF-Alpha Inhibitor Etanercept. Ann. Rheum. Dis. 2020, 79, 1251–1252. [Google Scholar] [CrossRef]
- Miyazawa, K.; Hondo, T.; Kanaya, T.; Tanaka, S.; Takakura, I.; Itani, W.; Rose, M.T.; Kitazawa, H.; Yamaguchi, T.; Aso, H. Characterization of Newly Established Bovine Intestinal Epithelial Cell Line. Histochem. Cell Biol. 2010, 133, 125–134. [Google Scholar] [CrossRef]
- Nienow, A.W.; Rafiq, Q.A.; Coopman, K.; Hewitt, C.J. A Potentially Scalable Method for the Harvesting of HMSCs from Microcarriers. Biochem. Eng. J. 2014, 85, 79–88. [Google Scholar] [CrossRef]
- Sulaiman, S.; Chowdhury, S.R.; Fauzi, M.B.; Rani, R.A.; Mohamadyahaya, N.H.; Tabata, Y.; Hiraoka, Y.; Idrus, R.B.H.; Hwei, N.M. 3D Culture of MSCs on a Gelatin Microsphere in a Dynamic Culture System Enhances Chondrogenesis. Int. J. Mol. Sci. 2020, 21, 2688. [Google Scholar] [CrossRef]







| Basal Media | Serum | Supplements |
|---|---|---|
| Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco, San Diego, CA, USA) | Foetal Bovine Serum (Sigma-Aldrich, St. Louis, MO, USA) | 1% GlutaMax (Gibco, San Diego, CA, USA) + 1% Antibiotic & Antimycotic (Corning, NY, USA) |
| Human Platelet Lysate (Merck, Rahway, NJ, USA) | ||
| Human Serum (Sigma-Aldrich, St. Louis, MO, USA) | ||
| DMEM-Knockout (KO) (Gibco, San Diego, CA, USA) | Foetal Bovine Serum (Sigma-Aldrich, St. Louis, MO, USA) | |
| Human Platelet Lysate (Merck, Rahway, NJ, USA) | ||
| Human Serum (Sigma-Aldrich, St. Louis, MO, USA) | ||
| DMEM Low Glucose (LG) (Sigma-Aldrich, St. Louis, MO, USA) | Foetal Bovine Serum (Sigma-Aldrich, St. Louis, MO, USA | |
| Human Platelet Lysate (Merck, Rahway, NJ, USA) | ||
| Human Serum (Sigma-Aldrich, St. Louis, MO, USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, B.; Sulaiman, N.; Fauzi, M.B.; Law, J.X.; Ng, M.H.; Yuan, T.L.; Azurah, A.G.N.; Mohd Yunus, M.H.; Idrus, R.B.H.; Yazid, M.D. A Three-Dimensional Xeno-Free Culture Condition for Wharton’s Jelly-Mesenchymal Stem Cells: The Pros and Cons. Int. J. Mol. Sci. 2023, 24, 3745. https://doi.org/10.3390/ijms24043745
Koh B, Sulaiman N, Fauzi MB, Law JX, Ng MH, Yuan TL, Azurah AGN, Mohd Yunus MH, Idrus RBH, Yazid MD. A Three-Dimensional Xeno-Free Culture Condition for Wharton’s Jelly-Mesenchymal Stem Cells: The Pros and Cons. International Journal of Molecular Sciences. 2023; 24(4):3745. https://doi.org/10.3390/ijms24043745
Chicago/Turabian StyleKoh, Benson, Nadiah Sulaiman, Mh Busra Fauzi, Jia Xian Law, Min Hwei Ng, Too Lih Yuan, Abdul Ghani Nur Azurah, Mohd Heikal Mohd Yunus, Ruszymah Bt Hj Idrus, and Muhammad Dain Yazid. 2023. "A Three-Dimensional Xeno-Free Culture Condition for Wharton’s Jelly-Mesenchymal Stem Cells: The Pros and Cons" International Journal of Molecular Sciences 24, no. 4: 3745. https://doi.org/10.3390/ijms24043745