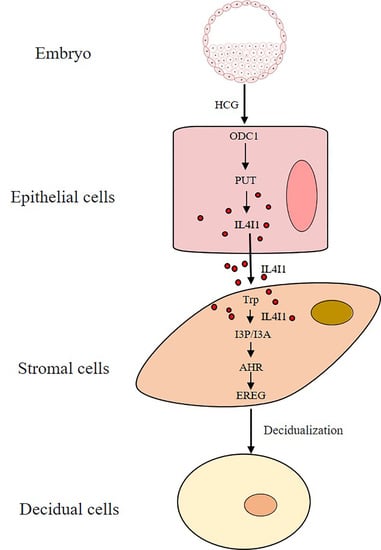
Human Chorionic Gonadotropin-Stimulated Interleukin-4-Induced-1 (IL4I1) Promotes Human Decidualization via Aryl Hydrocarbon Receptor
Abstract
:1. Introduction
2. Results
2.1. Effects of I3P on Human In Vitro Decidualization
2.2. I3P Stimulates Human In Vitro Decidualization by Activating AHR
2.3. Effects of I3A on Human In Vitro Decidualization
2.4. I3A Promotes Human In Vitro Decidualization by Activating AHR
2.5. I3P and I3A Regulate Human In Vitro Decidualization via AHR-EREG Pathway
2.6. IL4I1 Expression and Secretion Is Promoted by HCG
2.7. HCG Regulates IL4I1 Expression through Polyamine Metabolism
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Treatment of Human Endometrial Cells
4.3. RNA Extraction and Real-Time PCR
4.4. Western Blot
4.5. Nuclear and Cytoplasmic Fractions
4.6. Immunofluorescence
4.7. PUT Measurement by ELISA
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.H.; Liang, X.; Wang, T.S.; Liang, X.H.; Zuo, R.J.; Deng, W.B.; Zhang, Z.R.; Qin, F.N.; Zhao, Z.A.; Yang, Z.M. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) induction on Snail expression during mouse decidualization. Mol. Cell Endocrinol. 2013, 381, 272–279. [Google Scholar] [CrossRef]
- Dey, S.K.; Lim, H.; Das, S.K.; Reese, J.; Paria, B.C.; Daikoku, T.; Wang, H. Molecular cues to implantation. Endocr. Rev. 2004, 25, 341–373. [Google Scholar] [CrossRef] [PubMed]
- Ramathal, C.Y.; Bagchi, I.C.; Taylor, R.N.; Bagchi, M.K. Endometrial decidualization: Of mice and men. Semin. Reprod. Med. 2010, 28, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Gellersen, B.; Brosens, J.J. Cyclic Decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.-B. The tryptophan utilization concept in pregnancy. Obstet. Gynecol. Sci. 2014, 57, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, H.; Bai, M.; Gao, J.; Wu, X.; Yin, Y. Redox properties of tryptophan metabolism and the concept of tryptophan use in pregnancy. Int. J. Mol. Sci. 2017, 18, 1595. [Google Scholar] [CrossRef]
- Laurent, L.; Deroy, K.; St-Pierre, J.; Côté, F.; Sanderson, J.T.; Vaillancourt, C. Human placenta expresses both peripheral and neuronal isoform of tryptophan hydroxylase. Biochimie 2017, 140, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Bolte, A.C.; van Geijn, H.P.; Dekker, G.A. Pathophysiology of preeclampsia and the role of serotonin. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 95, 12–21. [Google Scholar] [CrossRef]
- Fields, A.M.; Welle, K.; Ho, E.S.; Mesaros, C.; Susiarjo, M. Vitamin B6 deficiency disrupts serotonin signaling in pancreatic islets and induces gestational diabetes in mice. Commun. Biol. 2021, 4, 421. [Google Scholar] [CrossRef]
- Hoshi, M.; Osawa, Y.; Nakamoto, K.; Morita, N.; Yamamoto, Y.; Ando, T.; Tashita, C.; Nabeshima, T.; Saito, K. Kynurenine produced by indoleamine 2,3-dioxygenase 2 exacerbates acute liver injury by carbon tetrachloride in mice. Toxicology 2020, 438, 152458. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, K.; Higuchi, T.; Fujiwara, H.; Nakayama, T.; Egawa, H.; Itoh, K.; Fujii, S.; Fujita, J. Induction of tryptophan 2,3-dioxygenase in the mouse endometrium during implantation. Biochem. Biophys. Res. Commun. 2000, 274, 166–170. [Google Scholar] [CrossRef]
- Chang, R.-Q.; Li, D.-J.; Li, M.-Q. The role of indoleamine-2,3-dioxygenase in normal and pathological pregnancies. Am. J. Reprod. Immunol. 2018, 79, e12786. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.-B. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci. Rep. 2015, 35, e00261. [Google Scholar] [CrossRef]
- Iwahashi, N.; Yamamoto, M.; Nanjo, S.; Toujima, S.; Minami, S.; Ino, K. Downregulation of indoleamine 2, 3-dioxygenase expression in the villous stromal endothelial cells of placentas with preeclampsia. J. Reprod. Immunol. 2017, 119, 54–60. [Google Scholar] [CrossRef]
- Kudo, Y.; Boyd, C.A.R.; Sargent, I.L.; Redman, C.W. Decreased tryptophan catabolism by placental indoleamine 2,3-dioxygenase in preeclampsia. Am. J. Obstet. Gynecol. 2003, 188, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef]
- Avilla, M.N.; Malecki, K.M.C.; Hahn, M.E.; Wilson, R.H.; Bradfield, C.A. The Ah receptor: Adaptive metabolism, ligand diversity, and the Xenokine Model. Chem. Res. Toxicol. 2020, 33, 860–879. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.J.; De Castro, K.P.; Joshi, A.D.; Elferink, C.J. Canonical and non-canonical aryl hydrocarbon receptor signaling pathways. Curr. Opin. Toxicol. 2017, 2, 87–92. [Google Scholar] [CrossRef]
- Gomez-Duran, A.; Ballestar, E.; Carvajal-Gonzalez, J.M.; Marlowe, J.L.; Puga, A.; Esteller, M.; Fernandez-Salguero, P.M. Recruitment of CREB1 and histone deacetylase 2 (HDAC2) to the mouse Ltbp-1 promoter regulates its constitutive expression in a dioxin receptor-dependent manner. J. Mol. Biol. 2008, 380, 1–16. [Google Scholar] [CrossRef]
- Shinde, R.; McGaha, T.L. The aryl hydrocarbon receptor: Connecting immunity to the microenvironment. Trends Immunol. 2018, 39, 1005–1020. [Google Scholar] [CrossRef]
- Puga, A.; Ma, C.; Marlowe, J.L. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem. Pharmacol. 2009, 77, 713–722. [Google Scholar] [CrossRef]
- Hernández-Ochoa, I.; Karman, B.N.; Flaws, J.A. The role of the aryl hydrocarbon receptor in the female reproductive system. Biochem. Pharmacol. 2009, 77, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Miller, M.A.; Harper, P.A. In utero exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin induces amphiregulin gene expression in the developing mouse ureter. Toxicol. Sci. 2006, 94, 163–174. [Google Scholar] [CrossRef] [PubMed]
- John, K.; Lahoti, T.S.; Wagner, K.; Hughes, J.M.; Perdew, G.H. The Ah receptor regulates growth factor expression in head and neck squamous cell carcinoma cell lines. Mol. Carcinog. 2014, 53, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Barnea, E.R.; Kirk, D.; Paidas, M.J. Preimplantation factor (PIF) promoting role in embryo implantation: Increases endometrial integrin-α2β3, amphiregulin and epiregulin while reducing betacellulin expression via MAPK in decidua. Reprod. Biol. Endocrinol. 2012, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Li, J.; Song, S.; Wang, J.; Cai, W.; Hu, W.; Ji, J.; Zhu, Z.; Zang, L.; Yan, R.; et al. A positive feedback between IDO1 metabolite and COL12A1 via MAPK pathway to promote gastric cancer metastasis. J. Exp. Clin. Cancer Res. 2019, 38, 314. [Google Scholar] [CrossRef]
- Chen, J.Y.; Li, C.F.; Kuo, C.C.; Tsai, K.K.; Hou, M.F.; Hung, W.C. Cancer/stroma interplay via cyclooxygenase-2 and indoleamine 2,3-dioxygenase promotes breast cancer progression. Breast Cancer Res. 2014, 16, 410. [Google Scholar] [CrossRef]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef]
- Novikov, O.; Wang, Z.; Stanford, E.A.; Parks, A.J.; Ramirez-Cardenas, A.; Landesman, E.; Laklouk, I.; Sarita-Reyes, C.; Gusenleitner, D.; Li, A.; et al. An aryl hydrocarbon receptor-mediated amplification loop that enforces cell migration in ER-/PR-/Her2- human breast cancer cells. Mol. Pharmacol. 2016, 90, 674–688. [Google Scholar] [CrossRef]
- D’Amato, N.C.; Rogers, T.J.; Gordon, M.A.; Greene, L.I.; Cochrane, D.R.; Spoelstra, N.S.; Nemkov, T.G.; D’Alessandro, A.; Hansen, K.C.; Richer, J.K. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 2015, 75, 4651–4664. [Google Scholar] [CrossRef] [Green Version]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Lemos, H.; Huang, L.; Prendergast, G.C.; Mellor, A.L. Immune control by amino acid catabolism during tumorigenesis and therapy. Nat. Rev. Cancer 2019, 19, 162–175. [Google Scholar] [CrossRef]
- Wang, Z.; Li, T.; Mao, C.; Liu, W.; Tao, Y. IL4I1-driven AHR signature: A new avenue for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 118. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, D.; Pires, A.C.S.; Guillemin, G.J.; Gilot, D. Detrimental activation of AhR pathway in cancer: An overview of therapeutic strategies. Curr. Opin. Immunol. 2021, 70, 15–26. [Google Scholar] [CrossRef]
- Boulland, M.-L.; Marquet, J.; Molinier-Frenkel, V.; Möller, P.; Guiter, C.; Lasoudris, F.; Copie-Bergman, C.; Baia, M.; Gaulard, P.; Leroy, K.; et al. Human IL4I1 is a secreted l-phenylalanine oxidase expressed by mature dendritic cells that inhibits T-lymphocyte proliferation. Blood 2007, 110, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Sadik, A.; Patterson, L.F.S.; Öztürk, S.; Mohapatra, S.R.; Panitz, V.; Secker, P.F.; Pfänder, P.; Loth, S.; Salem, H.; Prentzell, M.T.; et al. IL4I1 Is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell 2020, 182, 1252–1270. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.C.; Chen, S.T.; Hong, Z.K.; Li, S.Y.; Yang, Z.S.; Quan, S.; Yang, Z.M. Tryptophan and kynurenine stimulate human decidualization via activating Aryl hydrocarbon receptor: Short title: Kynurenine action on human decidualization. Reprod. Toxicol. 2020, 96, 282–292. [Google Scholar] [CrossRef]
- Tseng, L.; Gao, J.G.; Chen, R.; Zhu, H.H.; Mazella, J.; Powell, D.R. Effect of progestin, antiprogestin, and relaxin on the accumulation of prolactin and insulin-like growth factor-binding protein-1 messenger ribonucleic acid in human endometrial stromal cells. Biol. Reprod. 1992, 47, 441–450. [Google Scholar] [CrossRef]
- Takano, M.; Lu, Z.; Goto, T.; Fusi, L.; Higham, J.; Francis, J.; Withey, A.; Hardt, J.; Cloke, B.; Stavropoulou, A.V.; et al. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol. Endocrinol. 2007, 21, 2334–2349. [Google Scholar] [CrossRef]
- Khan, A.; Gamble, L.D.; Upton, D.H.; Ung, C.; Yu, D.M.T.; Ehteda, A.; Pandher, R.; Mayoh, C.; Hébert, S.; Jabado, N.; et al. Dual targeting of polyamine synthesis and uptake in diffuse intrinsic pontine gliomas. Nat. Commun. 2021, 12, 971. [Google Scholar] [CrossRef]
- Imbesi, R.; Castrogiovanni, P. Embryonic and postnatal development in experimental tryptophan deprived rats. A preliminary study. J. Mol. Histol. 2008, 39, 487–498. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed]
- Santillan, M.K.; Pelham, C.J.; Ketsawatsomkron, P.; Santillan, D.A.; Davis, D.R.; Devor, E.J.; Gibson-Corley, K.N.; Scroggins, S.M.; Grobe, J.L.; Yang, B.; et al. Pregnant mice lacking indoleamine 2,3-dioxygenase exhibit preeclampsia phenotypes. Physiol. Rep. 2015, 3, e12257. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-D.; Gao, Y.-J.; Tian, X.-C.; Yang, Z.-Q.; Cao, H.; Zhang, Q.-L.; Guo, B.; Yue, Z.-P. Differential expression and regulation of Tdo2 during mouse decidualization. J. Endocrinol. 2014, 220, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Dupont, V.; Berg, A.H.; Yamashita, M.; Huang, C.; Covarrubias, A.E.; Ali, S.; Stotland, A.; Van Eyk, J.E.; Jim, B.; Thadhani, R.; et al. Impaired renal reserve contributes to preeclampsia via the kynurenine and soluble fms–like tyrosine kinase 1 pathway. J. Clin. Investig. 2022, 132, e158346. [Google Scholar] [CrossRef] [PubMed]
- Torti, M.F.; Giovannoni, F.; Quintana, F.J.; García, C.C. The Aryl Hydrocarbon Receptor as a modulator of anti-viral immunity. Front. Immunol. 2021, 12, 624293. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.E.; Bott, D.; Ahmed, S.; Hutin, D.; Gomez, A.; Tamblyn, L.; Zhou, A.C.; Watts, T.H.; Grant, D.M.; Matthews, J. 3-Methylcholanthrene induces Chylous ascites in TCDD-inducible poly-ADP-ribose polymerase (tiparp) knockout mice. Int. J. Mol. Sci. 2019, 20, 2312. [Google Scholar] [CrossRef]
- Trikha, P.; Lee, D.A. The role of AhR in transcriptional regulation of immune cell development and function. Biochim. Biophys. Acta (BBA) Rev. Cancer 2020, 1873, 188335. [Google Scholar] [CrossRef]
- Rataj, F.; Möller, F.J.; Jähne, M.; Zierau, O.; Diel, P.; Vollmer, G.; Kretzschmar, G. Regulation of uterine AHR battery gene expression by 17β-Estradiol is predominantly mediated by estrogen receptor α. Arch. Toxicol. 2012, 86, 1603–1612. [Google Scholar] [CrossRef]
- Rataj, F.; Möller, F.J.; Jähne, M.; Hönscheid, P.; Zierau, O.; Vollmer, G.; Kretzschmar, G. Progesterone, as well as 17β-estradiol, is important for regulating AHR battery homoeostasis in the rat uterus. Arch. Toxicol. 2015, 89, 393–404. [Google Scholar] [CrossRef]
- Abbott, B.D.; Schmid, J.E.; Pitt, J.A.; Buckalew, A.R.; Wood, C.R.; Held, G.A.; Diliberto, J.J. Adverse reproductive outcomes in the transgenic Ah receptor-deficient mouse. Toxicol. Appl. Pharmacol. 1999, 155, 62–70. [Google Scholar] [CrossRef]
- Baba, T.; Mimura, J.; Nakamura, N.; Harada, N.; Yamamoto, M.; Morohashi, K.-I.; Fujii-Kuriyama, Y. Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol. Cell. Biol. 2005, 25, 10040–10051. [Google Scholar] [CrossRef]
- Islam, M.R.; Ikeguchi, Y.; Yamagami, K.; El-Sharawy, M.; Yamauchi, N. Development of an in vitro model to study uterine functions and early implantation using rat uterine explants. Cell Tissue Res. 2017, 370, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Das, N.; Wang, J.; Lim, H.; Schryver, B.; Plowman, G.D.; Dey, S.K. Expression of betacellulin and epiregulin genes in the mouse uterus temporally by the blastocyst solely at the site of its apposition is coincident with the “window” of implantation. Dev. Biol. 1997, 190, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Umehara, T.; Hoshino, Y. Roles of epidermal growth factor (EGF)-like factor in the ovulation process. Reprod. Med. Biol. 2016, 15, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Marquet, J.; Lasoudris, F.; Cousin, C.; Puiffe, M.L.; Martin-Garcia, N.; Baud, V.; Chereau, F.; Farcet, J.P.; Molinier-Frenkel, V.; Castellano, F. Dichotomy between factors inducing the immunosuppressive enzyme IL-4-induced gene 1 (IL4I1) in B lym-phocytes and mononuclear phagocytes. Eur. J. Immunol. 2010, 40, 2557–2568. [Google Scholar] [CrossRef]
- Park, S.H.; Kang, K.; Giannopoulou, E.; Qiao, Y.; Kang, K.; Kim, G.; Park-Min, K.-H.; Ivashkiv, L.B. Type I interferons and the cytokine TNF cooperatively reprogram the macrophage epigenome to promote inflammatory activation. Nat. Immunol. 2017, 18, 1104–1116. [Google Scholar] [CrossRef]
- Mazzoni, A.; Capone, M.; Ramazzotti, M.; Vanni, A.; Locatello, L.G.; Gallo, O.; De Palma, R.; Cosmi, L.; Liotta, F.; Annunziato, F.; et al. IL4I1 is expressed by head-neck cancer-derived mesenchymal stromal cells and contributes to suppress T cell proliferation. J. Clin. Med. 2021, 10, 2111. [Google Scholar] [CrossRef]
- He, B.; Zhang, H.; Wang, J.; Liu, M.; Sun, Y.; Guo, C.; Lu, J.; Wang, H.; Kong, S. Blastocyst activation engenders transcriptome reprogram affecting X-chromosome reactivation and inflammatory trigger of implantation. Proc. Natl. Acad. Sci. USA 2019, 116, 16621–16630. [Google Scholar] [CrossRef] [PubMed]
- Vialard, F.; El Sirkasi, M.; Tronchon, V.; Boudjenah, R.; Molina-Gomes, D.; Bergere, M.; Mauduit, C.; Wainer, R.; Selva, J.; Benahmed, M. Tumor necrosis factor-308 polymorphism increases the embryo implantation rate in women undergoing in vitro fertilization. Hum. Reprod. 2013, 28, 2774–2783. [Google Scholar] [CrossRef] [Green Version]
- Hay, D.L.; Lopata, A. Chorionic gonadotropin secretion by human embryos in vitro. J. Clin. Endocrinol. Metab. 1988, 67, 1322–1324. [Google Scholar] [CrossRef] [PubMed]
- Makrigiannakis, A.; Vrekoussis, T.; Zoumakis, E.; Kalantaridou, S.N.; Jeschke, U. The role of HCG in implantation: A mini-review of molecular and clinical evidence. Int. J. Mol. Sci. 2017, 18, 1305. [Google Scholar] [CrossRef] [PubMed]
- Elsheimer-Matulova, M.; Polansky, O.; Seidlerova, Z.; Varmuzova, K.; Stepanova, H.; Fedr, R.; Rychlik, I. Interleukin 4 inducible 1 gene (IL4I1) is induced in chicken phagocytes by salmonella enteritidis infection. Vet. Res. 2020, 51, 67. [Google Scholar] [CrossRef]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef]
- Fenelon, J.C.; Lefèvre, P.L.; Banerjee, A.; Murphy, B.D. Regulation of diapause in carnivores. Reprod. Domest. Anim. 2017, 52, 12–17. [Google Scholar] [CrossRef]
- Lenis, Y.Y.; Elmetwally, M.A.; Maldonado-Estrada, J.G.; Bazer, F.W. Physiological importance of polyamines. Zygote 2017, 25, 244–255. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Chi, Y.J.; Yu, Y.S.; Liu, J.L.; Su, R.W.; Ma, X.H.; Shan, C.H.; Yang, Z.M. Polyamines are essential in embryo implantation: Expression and function of polyamine-related genes in mouse uterus during peri-implantation period. Endocrinology 2008, 149, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Fenelon, J.C.; Murphy, B.D. Inhibition of polyamine synthesis causes entry of the mouse blastocyst into embryonic diapause. Biol. Reprod. 2017, 97, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.W.; Yang, Y.; Li, T.; Chen, Z.C.; Fu, T.; Pan, J.M.; Ou, J.P.; Yang, Z.M. ATP mediates the interaction between human blastocyst and endometrium. Cell Prolif. 2020, 53, e12737. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-S.; Pan, H.-Y.; Shi, W.-W.; Chen, S.-T.; Wang, Y.; Li, M.-Y.; Zhang, H.-Y.; Yang, C.; Liu, A.-X.; Yang, Z.-M. Regulation and function of laminin A5 during mouse and human decidualization. Int. J. Mol. Sci. 2021, 23, 199. [Google Scholar] [CrossRef]
- Zuo, R.-J.; Gu, X.-W.; Qi, Q.; Wang, T.-S.; Zhao, X.-Y.; Liu, J.-L.; Yang, Z.-M. Warburg-like glycolysis and lactate shuttle in mouse decidua during early pregnancy. J. Biol. Chem. 2015, 290, 21280–21291. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Yan, J.Q.; Song, Z.; Liu, Y.F.; Song, M.J.; Qin, J.W.; Yang, Z.M.; Liang, X.H. Molecular characterization of lysyl oxidase-mediated extracellular matrix remodeling during mouse decidualization. FEBS Lett. 2017, 591, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Song, Z.; Yan, Y.P.; Li, B.; Song, M.J.; Liu, Y.F.; Yang, Z.S.; Li, M.Y.; Liu, A.X.; Quan, S.; et al. Aldosterone from endometrial glands is benefit for human decidualization. Cell Death Dis. 2020, 11, 679. [Google Scholar] [CrossRef] [PubMed]







| Genes | Primers (5′–3′) | Accession Number | Size (bp) |
|---|---|---|---|
| CYP1A1 (human) | GTGCGGCAGGGCGATGATTT GGCTGAAGGACATGCTCTGACC | NM_000499.4 | 85 |
| CYP1B1 (human) | AACCGCAACTTCAGCAACTT GAGGATAAAGGCGTCCATCA | NM_000104.3 | 102 |
| EREG (human) | GTGTGGCTCAAGTGTCAATAAC GGAACCGACGACTGTGATAAG | NM_001432.3 | 233 |
| FOXO1 (human) | CGAGCTGCCAAGAAGAAA TTCGAGGGCGAAATGTAC | NM_00201 | 105 |
| IGFBP1 (human) | CCAAACTGCAACAAGAATG GTAGACGCACCAGCAGAG | NM_001,013,029 | 87 |
| PRL (human) | AAGCTGTAGAGATTGAGGAGCAAA TCAGGATGAACCTGGCTGACTA | NM_000948 | 76 |
| RPL7 (human) | CTGCTGTGCCAGAAACCCTT TCTTGCCATCCTCGCCAT | NM_000971 | 194 |
| TIPARP (human) | TGAGCCAGACTGTGTAGTGC AACCCCATCAAGTGAGCCAG | NM_001184718.2 | 193 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.-M.; Zhang, T.-T.; He, Y.-Y.; Luo, H.-N.; Hong, Y.-Q.; Yang, Z.-M. Human Chorionic Gonadotropin-Stimulated Interleukin-4-Induced-1 (IL4I1) Promotes Human Decidualization via Aryl Hydrocarbon Receptor. Int. J. Mol. Sci. 2023, 24, 3163. https://doi.org/10.3390/ijms24043163
Luo J-M, Zhang T-T, He Y-Y, Luo H-N, Hong Y-Q, Yang Z-M. Human Chorionic Gonadotropin-Stimulated Interleukin-4-Induced-1 (IL4I1) Promotes Human Decidualization via Aryl Hydrocarbon Receptor. International Journal of Molecular Sciences. 2023; 24(4):3163. https://doi.org/10.3390/ijms24043163
Chicago/Turabian StyleLuo, Jia-Mei, Tong-Tong Zhang, Yu-Ying He, Hui-Na Luo, Yu-Qi Hong, and Zeng-Ming Yang. 2023. "Human Chorionic Gonadotropin-Stimulated Interleukin-4-Induced-1 (IL4I1) Promotes Human Decidualization via Aryl Hydrocarbon Receptor" International Journal of Molecular Sciences 24, no. 4: 3163. https://doi.org/10.3390/ijms24043163