Gestational Diabetes Mellitus and Small-for-Gestational-Age: An Insight into the Placental Molecular Biomarkers
Abstract
:1. Introduction
2. Results
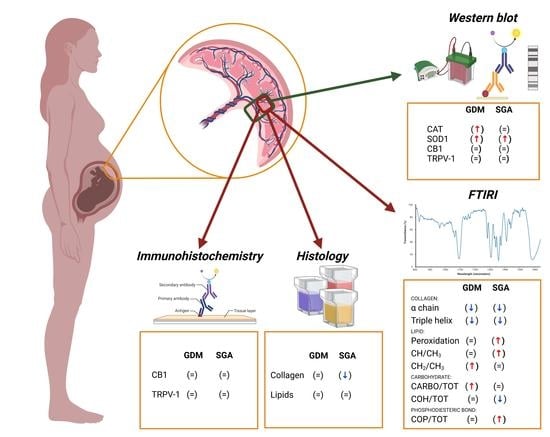
2.1. GDM and SGA Correlate with Increased Levels of Antioxidant Enzymes in Term Placenta
2.2. Fourier Transform Infrared Imaging (FTIR) Analysis of CTRL, GDM, and SGA Placentae
2.3. SGA and GDM Alter Collagen Structures, Whereas Only SGA Decreases Collagen Deposition in Placental CV
2.4. GDM and SGA Modify the Lipidic Composition of Placental CVs
2.5. The Carbohydrate Content Is Increased in the CVs of GDM Placentae
2.6. SGA Placental CVs Are Characterized by Lower Glycosylated Compounds and Higher Phosphodiester Bonds
2.7. GDM and SGA Do Not Alter the Placental Levels of Endocannabinoid Receptors CB1 and TRPV-1
3. Discussion
4. Materials and Methods
4.1. Ethics Declarations and Sample Collection
4.2. Evaluation of Protein Levels by Western Blot
4.3. Immunohistochemistry and Image Analysis
4.4. Histological Analysis of Collagen and Lipid Content
4.5. FTIRI Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaughan, O.R.; Fowden, A.L. Placental metabolism: Substrate requirements and the response to stress. Reprod. Domest. Anim. 2016, 51, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and function of the normal human placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Behboudi-Gandevani, S.; Amiri, M.; Bidhendi Yarandi, R.; Ramezani Tehrani, F. The impact of diagnostic criteria for gestational diabetes on its prevalence: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Practice, C. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization Guideline. Diabetes Res. Clin. Pract. 2014, 103, 341–363. [Google Scholar] [CrossRef]
- Alia, S.; Pugnaloni, S.; Borroni, F.; Mazzanti, L.; Giannubilo, S.R.; Ciavattini, A.; Vignini, A. Impact of gestational diabetes mellitus in maternal and fetal health: An update. Diabetes Updates 2019, 5, 1–6. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The pathophysiology of gestational diabetes mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [Green Version]
- The American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef] [Green Version]
- Associazione Medici Diabetologi (AMD); Società Italiana di Diabetologia (SID). Standard Italiani per la Cura del Diabete Mellito; AMD: Rome, Italy, 2018; pp. 118–127. [Google Scholar]
- De Onis, M.; Habicht, J.P. Anthropometric reference data for international use: Recommendations from a World Health Organization Expert Committee. Am. J. Clin. Nutr. 1996, 64, 650–658. [Google Scholar] [CrossRef] [Green Version]
- Small-for-Gestational-Age (SGA) Newborns—Children’s Health Issues—MSD Manual Consumer Version. (n.d.) Available online: https://www.msdmanuals.com/home/children-s-health-issues/general-problems-in-newborns/small-for-gestational-age-sga-newborn (accessed on 10 November 2022).
- Osuchukwu, O.O.; Reed, D.J. Small for Gestational Age. StatPearls. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563247/ (accessed on 10 November 2022).
- Tamaro, G.; Pizzul, M.; Gaeta, G.; Servello, R.; Trevisan, M.; Bohm, P.; Materassi, P.M.A.; Macaluso, A.; Valentini, D.; Pellegrin, M.C.; et al. Prevalence of children born small for gestational age with short stature who qualify for growth hormone treatment: A preliminary population-based study. Horm. Res. Paediatr. 2019, 91, 308. Available online: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L630602200%0A (accessed on 10 November 2022). [CrossRef]
- Nawathe, A.R.; Christian, M.; Kim, S.H.; Johnson, M.; Savvidou, M.D.; Terzidou, V. Insulin-like growth factor axis in pregnancies affected by fetal growth disorders. Clin. Epigenet. 2016, 8, 11. [Google Scholar] [CrossRef]
- Baker, B.C.; Mackie, F.L.; Lean, S.C.; Greenwood, S.L.; Heazell, A.E.P.; Forbes, K.; Jones, R.L. Placental dysfunction is associated with altered microRNA expression in pregnant women with low folate status. Mol. Nutr. Food Res. 2017, 61, 1600646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosco Becerra, C.; Díaz Guerra, E.; Gutierrez Rojas, R.; González Montero, J.; Parra Cordero, M.; Ramón Rodrigo Salinas, R.; Barja Yañez, P. Placental Hypoxia Developed During Preeclampsia Induces Telocytes Apoptosis in Chorionic Villi Affecting the Maternal-Fetus Metabolic Exchange. Curr. Stem Cell Res. Ther. 2016, 11, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Wong, I.; Moller, A.; Giachini, F.R.; Lima, V.V.; Toledo, F.; Stojanova, J.; Sobrevia, L.; San Martín, S. Placental structure in gestational diabetes mellitus. Biochim. Biophys. Acta—Mol. Basis Dis. 2020, 1866, 165535. [Google Scholar] [CrossRef] [PubMed]
- Valent, A.M.; Choi, H.; Kolahi, K.S.; Thornburg, K.L. Hyperglycemia and gestational diabetes suppress placental glycolysis and mitochondrial function and alter lipid processing. FASEB J. 2021, 35, e21423. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M.; Hiden, U.; Desoye, G.; Froehlich, J.; De Mouzon, S.H.; Jawerbaum, A. The Role of Oxidative Stress in the Pathophysiology of Gestational Diabetes Mellitus. Antioxid. Redox Signal. 2011, 15, 3061–3100. [Google Scholar] [CrossRef]
- Gómez-Vilarrubla, A.; Mas-Parés, B.; Díaz, M.; Xargay-Torrent, S.; Carreras-Badosa, G.; Jové, M.; Martin-Gari, M.; Bonmatí-Santané, A.; de Zegher, F.; Ibañez, L.; et al. Fatty acids in the placenta of appropiate- versus small-for-gestational-age infants at term birth. Placenta 2021, 109, 4–10. [Google Scholar] [CrossRef]
- Freitas, H.R.; Isaac, A.R.; Malcher-Lopes, R.; Diaz, B.L.; Trevenzoli, I.H.; De Melo Reis, R.A. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr. Neurosci. 2017, 21, 695–714. [Google Scholar] [CrossRef]
- Maia, J.; Fonseca, B.M.; Teixeira, N.; Correia-Da-Silva, G. The fundamental role of the endocannabinoid system in endometrium and placenta: Implications in pathophysiological aspects of uterine and pregnancy disorders. Hum. Reprod. Update 2020, 26, 586–602. [Google Scholar] [CrossRef]
- Lombó, M.; Giommi, C.; Paolucci, M.; Notarstefano, V.; Montik, N.; Delli Carpini, G.; Ciavattini, A.; Ragusa, A.; Maradonna, F.; Giorgini, E.; et al. Preeclampsia Correlates with an Increase in Cannabinoid Receptor 1 Levels Leading to Macromolecular Alterations in Chorionic Villi of Term Placenta. Int. J. Mol. Sci. 2022, 23, 12931. [Google Scholar] [CrossRef]
- Bonnier, F.; Byrne, H.J. Understanding the molecular information contained in principal component analysis of vibrational spectra of biological systems. Analyst 2011, 137, 322–332. [Google Scholar] [CrossRef]
- Notarstefano, V.; Sabbatini, S.; Conti, C.; Pisani, M.; Astolfi, P.; Pro, C.; Rubini, C.; Vaccari, L.; Giorgini, E. Investigation of human pancreatic cancer tissues by Fourier Transform Infrared Hyperspectral Imaging. J. Biophotonics 2020, 13, e201960071. [Google Scholar] [CrossRef] [PubMed]
- Notarstefano, V.; Belloni, A.; Sabbatini, S.; Pro, C.; Orilisi, G.; Monterubbianesi, R.; Tosco, V.; Byrne, H.J.; Vaccari, L.; Giorgini, E. Cytotoxic Effects of 5-Azacytidine on Primary Tumour Cells and Cancer Stem Cells from Oral Squamous Cell Carcinoma: An In Vitro FTIRM Analysis. Cells 2021, 10, 2127. [Google Scholar] [CrossRef] [PubMed]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2016, 52, 456–506. [Google Scholar] [CrossRef]
- Stani, C.; Vaccari, L.; Mitri, E.; Birarda, G. FTIR investigation of the secondary structure of type I collagen: New insight into the amide III band. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 118006. [Google Scholar] [CrossRef] [PubMed]
- Licini, C.; Notarstefano, V.; Marchi, S.; Cerqueni, G.; Ciapetti, G.; Vitale-Brovarone, C.; Giorgini, E.; Mattioli-Belmonte, M. Altered type I collagen networking in osteoporotic human femoral head revealed by histomorphometric and Fourier transform infrared imaging correlated analyses. Biofactors 2022, 48, 1089–1110. [Google Scholar] [CrossRef] [PubMed]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef]
- Belloni, A.; Furlani, M.; Greco, S.; Notarstefano, V.; Pro, C.; Randazzo, B.; Pellegrino, P.; Zannotti, A.; Carpini, G.D.; Ciavattini, A.; et al. Uterine leiomyoma as useful model to unveil morphometric and macromolecular collagen state and impairment in fibrotic diseases: An ex-vivo human study. Biochim. Biophys. Acta—Mol. Basis Dis. 2022, 1868, 166494. [Google Scholar] [CrossRef]
- Pujal, J.M.; Roura, S.; Muñoz-Marmol, A.M.; Mate, J.L.; Bayes-Genis, A. Fetal-maternal interface: A chronicle of allogeneic coexistence. Chimerism 2012, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wu, J.; Yang, L.; Wang, H.; Xu, Y.; Shen, K. Fourier Transform Infrared Spectroscopy: An Innovative Method for the Diagnosis of Ovarian Cancer. Cancer Manag. Res. 2021, 13, 2389. [Google Scholar] [CrossRef]
- Burton, G.J.; Sebire, N.J.; Myatt, L.; Tannetta, D.; Wang, Y.L.; Sadovsky, Y.; Staff, A.C.; Redman, C.W. Optimising sample collection for placental research. Placenta 2014, 35, 9–22. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, E.H.; Kim, Y.R.; Kim, N.; Jung, J.E.; Han, S.H.; Cho, H.Y. Effect of Endogenic and Exogenic Oxidative Stress Triggers on Adverse Pregnancy Outcomes: Preeclampsia, Fetal Growth Restriction, Gestational Diabetes Mellitus and Preterm Birth. Int. J. Mol. Sci. 2021, 22, 10122. [Google Scholar] [CrossRef] [PubMed]
- Prins, J.R.; Schoots, M.H.; Wessels, J.I.; Campmans-Kuijpers, M.J.E.; Navis, G.J.; van Goor, H.; Robertson, S.A.; van der Beek, E.M.; Sobrevia, L.; Gordijn, S.J. The influence of the dietary exposome on oxidative stress in pregnancy complications. Mol. Asp. Med. 2022, 87, 101098. [Google Scholar] [CrossRef]
- Biri, A.; Onan, A.; Devrim, E.; Babacan, F.; Kavutcu, M.; Durak, I. Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta 2006, 27, 327–332. [Google Scholar] [CrossRef]
- Wang, S.; Ning, J.; Huai, J.; Yang, H. Hyperglycemia in Pregnancy-Associated Oxidative Stress Augments Altered Placental Glucose Transporter 1 Trafficking via AMPKα/p38MAPK Signaling Cascade. Int. J. Mol. Sci. 2022, 23, 8572. [Google Scholar] [CrossRef]
- Coughlan, M.T.; Vervaart, P.P.; Permezel, M.; Georgiou, H.M.; Rice, G.E. Altered placental oxidative stress status in gestational diabetes mellitus. Placenta 2004, 25, 78–84. [Google Scholar] [CrossRef]
- Lopez-Tinoco, C.; Segundo, C.; Aguilar-Diosdado, M. Gestational diabetes and oxidative stress. Curr. Trends Endocrinol. 2014, 7, 45–55. [Google Scholar]
- Díaz, M.; Aragonés, G.; Sánchez-Infantes, D.; Bassols, J.; Pérez-Cruz, M.; De Zegher, F.; Lopez-Bermejo, A.; Ibáñez, L. Mitochondrial DNA in Placenta: Associations with Fetal Growth and Superoxide Dismutase Activity. Horm. Res. Paediatr. 2014, 82, 303–309. [Google Scholar] [CrossRef]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, Ö.; Durak, I. Role of oxidative stress in intrauterine growth restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef]
- Pielesz, A.; Biniaś, D.; Waksmańska, W.; Bobiński, R. Lipid bands of approx. 1740 cm−1 as spectral biomarkers and image of tissue oxidative stress. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 286, 121926. [Google Scholar] [CrossRef]
- Powell, T.L.; Jansson, T.; Illsley, N.P.; Wennergren, M.; Korotkova, M.; Strandvik, B. Composition and permeability of syncytiotrophoblast plasma membranes in pregnancies complicated by intrauterine growth restriction. Biochim. Biophys. Acta—Biomembr. 1999, 1420, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Santillan, J.A.; Lazo-de-la-Vega-Monroy, M.L.; Rodriguez-Saldaña, G.C.; Solis-Barbosa, M.A.; Corona-Figueroa, M.A.; Gonzalez-Dominguez, M.I.; Gomez-Zapata, H.M.; Malacara, J.M.; Barbosa-Sabanero, G. Placental Nutrient Transporters and Maternal Fatty Acids in SGA, AGA, and LGA Newborns From Mothers With and Without Obesity. Front. Cell Dev. Biol. 2022, 10, 559. [Google Scholar] [CrossRef] [PubMed]
- Roig-Pérez, S.; Guardiola, F.; Moretó, M.; Ferrer, R. Lipid peroxidation induced by DHA enrichment modifies paracellular permeability in Caco-2 cells: Protective role of taurine. J. Lipid Res. 2004, 45, 1418–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassen, S.S.; Zemski-Berry, K.; Raymond-Whish, S.; Driver, C.; Hobbins, J.C.; Powell, T.L. Altered Cord Blood Lipid Concentrations Correlate with Birth Weight and Doppler Velocimetry of Fetal Vessels in Human Fetal Growth Restriction Pregnancies. Cells 2022, 11, 3110. [Google Scholar] [CrossRef] [PubMed]
- Lazo-De-La-Vega-Monroy, M.L.; Mata-Tapia, K.A.; Garcia-Santillan, J.A.; Corona-Figueroa, M.A.; Gonzalez-Dominguez, M.I.; Gomez-Zapata, H.M.; Malacara, J.M.; Daza-Benitez, L.; Barbosa-Sabanero, G. Association of placental nutrient sensing pathways with birth weight. Reproduction 2020, 160, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Pan, Z.; Guo, F.; Wang, H.; Long, W.; Wang, H.; Yu, B. Placental metabolic profiling in gestational diabetes mellitus: An important role of fatty acids. J. Clin. Lab. Anal. 2021, 35, e24096. [Google Scholar] [CrossRef]
- Cetin, I.; Alvino, G.; Cardellicchio, M. Long chain fatty acids and dietary fats in fetal nutrition. J. Physiol. 2009, 587, 3441. [Google Scholar] [CrossRef]
- Castillo-Castrejon, M.; Powell, T.L. Placental Nutrient Transport in Gestational Diabetic Pregnancies. Front. Endocrinol. 2017, 8, 306. [Google Scholar] [CrossRef] [Green Version]
- Vettor, R.; Pagano, C. The role of the endocannabinoid system in lipogenesis and fatty acid metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 51–63. [Google Scholar] [CrossRef]
- Choi, S.I.; Yoo, S.; Lim, J.Y.; Hwang, S.W. Are Sensory TRP Channels Biological Alarms for Lipid Peroxidation? Int. J. Mol. Sci. 2014, 15, 16430–16457. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, N.Y.; Shehata, M.H.; Gadallah, H.N.; Sayed, W.M.; Othman, A.A. Morphological and ultrastructural changes in the placenta of the diabetic pregnant Egyptian women. Acta Histochem. 2018, 120, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Chui, A.; Gunatillake, T.; Brennecke, S.P.; Ignjatovic, V.; Monagle, P.T.; Whitelock, J.M.; Van Zanten, D.E.; Eijsink, J.; Wang, Y.; Deane, J.; et al. Expression of Biglycan in First Trimester Chorionic Villous Sampling Placental Samples and Altered Function in Telomerase-Immortalized Microvascular Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1168–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chojkier, M.; Houglum, K.; Solis-Herruzo, J.; Brenner, D.A. Stimulation of collagen gene expression by ascorbic acid in cultured human fibroblasts. A role for lipid peroxidation? J. Biol. Chem. 1989, 264, 16957–16962. [Google Scholar] [CrossRef] [PubMed]









| CTRL | GDM | SGA | |
|---|---|---|---|
| Number of pregnant women | 12 | 12 | 12 |
| Parity | |||
| Multiparous | 9 (75%) | 5 (41.66%) | 6 (50%) |
| Primiparous | 3 (25%) | 7 (58.33%) | 6 (50%) |
| Mode of delivery (%) Natural birth Cesarean section | 6 (50%) 6 (50%) | 7 (58.33%) 5 (41.66%) | 5 (41.66%) 7 (58.33%) |
| Maternal age (years) | 34 ± 3.51 | 34.58 ± 5.08 | 32.25 ± 5.06 |
| Gestationl age (weeks) | 39.41 ± 1.50 | 38 ± 1.83 | 38 ± 2.46 |
| Fetal length (cm) * Fetal weight (g) | 50.08 ± 3.80 * 3410.08 ± 405.33 | 47.75 ± 2.80 * 2945.41 ± 631.73 | 46.66 ± 3.72 * 2209.33 ± 616.01 **** |
| OGTT test Time 0 (Fasting) Time 1 (1 h pgi) * Time 2 (2 h pgi) | 77.36 ± 9.58 115.54 ± 29.15 ** 102.36 ± 30.04 | 99.16 ± 20.36 ** 183.8 ± 33.43 **** 126.5 ± 45.10 ** | 81.75 ± 9.31 126.87 ± 36.82 ** 99.75 ± 35.45 |
| Fetal sex | |||
| Female | 6 (50%) | 8 (66.67%) | 8 (66.67%) |
| Male | 6 (50%) | 4 (33.33%) | 4 (33.33%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giommi, C.; Lombό, M.; Montik, N.; Paolucci, M.; Notarstefano, V.; Delli Carpini, G.; Ciavattini, A.; Ragusa, A.; Maradonna, F.; Giorgini, E.; et al. Gestational Diabetes Mellitus and Small-for-Gestational-Age: An Insight into the Placental Molecular Biomarkers. Int. J. Mol. Sci. 2023, 24, 2240. https://doi.org/10.3390/ijms24032240
Giommi C, Lombό M, Montik N, Paolucci M, Notarstefano V, Delli Carpini G, Ciavattini A, Ragusa A, Maradonna F, Giorgini E, et al. Gestational Diabetes Mellitus and Small-for-Gestational-Age: An Insight into the Placental Molecular Biomarkers. International Journal of Molecular Sciences. 2023; 24(3):2240. https://doi.org/10.3390/ijms24032240
Chicago/Turabian StyleGiommi, Christian, Marta Lombό, Nina Montik, Michela Paolucci, Valentina Notarstefano, Giovanni Delli Carpini, Andrea Ciavattini, Antonio Ragusa, Francesca Maradonna, Elisabetta Giorgini, and et al. 2023. "Gestational Diabetes Mellitus and Small-for-Gestational-Age: An Insight into the Placental Molecular Biomarkers" International Journal of Molecular Sciences 24, no. 3: 2240. https://doi.org/10.3390/ijms24032240