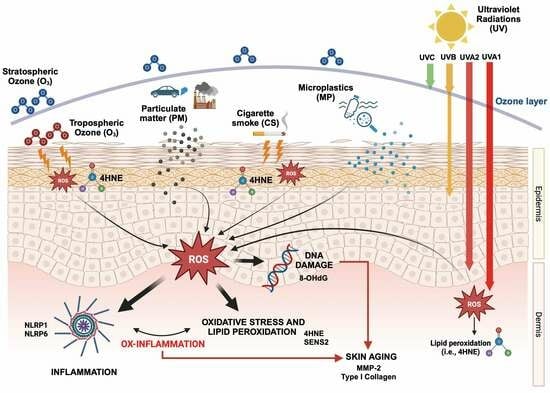
Comparison of Pollutant Effects on Cutaneous Inflammasomes Activation
Abstract
:1. Introduction
2. Results
2.1. Effect of Environmental Pollutants on Cutaneous Oxidative Stress and DNA Damage
2.2. Exposure to Air Pollutants Promotes Extrinsic Aging Markers
2.3. Air Pollutants Differently Trigger NLRs Inflammasomes Activation in Human Skin Biopsies
2.4. NLRP1 Most Susceptible Cutaneous Inflammasome to Air Pollutants Exposure
2.5. Air Pollutant Exposure Induced the Activation of Cutaneous Inflammasome and Pyroptosis Responses
3. Discussion
4. Materials and Methods
4.1. Skin Biopsies, Culture, and Treatments
4.2. Immunofluorescence Staining
4.3. RNA Extraction and Quantitative Real-Time PCR (rt-PCR)
4.4. Protein Extraction and Western Blotting
4.5. MMP-2 Zymography
4.6. ELISA for IL-1β
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valacchi, G.; Sticozzi, C.; Pecorelli, A.; Cervellati, F.; Cervellati, C.; Maioli, E. Cutaneous responses to environmental stressors. Ann. N. Y. Acad. Sci. 2012, 1271, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Woodby, B.; Penta, K.; Pecorelli, A.; Lila, M.A.; Valacchi, G. Skin Health from the Inside Out. Annu. Rev. Food Sci. Technol. 2020, 11, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Virgili, F.; Cervellati, C.; Pecorelli, A. OxInflammation: From subclinical condition to pathological biomarker. Front. Physiol. 2018, 9, 858. [Google Scholar] [CrossRef]
- Gurung, P.; Kanneganti, T.D. Autoinflammatory Skin Disorders: The Inflammasomme in Focus. Trends Mol. Med. 2016, 22, 545–564. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 461144. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yan, S.; Wu, M.; Li, F.; Xu, X.; Song, W.; Zhao, J.; Xu, J.; Kan, H. Ambient ozone pollution as a risk factor for skin disorders. Br. J. Dermatol. 2011, 165, 224–225. [Google Scholar] [CrossRef]
- Yee, M.S.L.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Wong, S.F.; Kok, Y.Y.; Tan, B.K.; Wong, C.Y.; Leong, C.O. Impact of microplastics and nanoplastics on human health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Magnani, N.D.; Muresan, X.M.; Belmonte, G.; Cervellati, F.; Sticozzi, C.; Pecorelli, A.; Miracco, C.; Marchini, T.; Evelson, P.; Valacchi, G. Skin damage mechanisms related to airborne particulate matter exposure. Toxicol. Sci. 2016, 149, 227–236. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, S.; Lee, S.W.; Jung, J.; Lee, S.H.; Na, H.W.; Kim, H.J.; Hong, Y.D.; Park, W.S.; Lee, T.G.; et al. Molecule-resolved visualization of particulate matter on human skin using multimodal nonlinear optical imaging. Int. J. Mol. Sci. 2021, 22, 5199. [Google Scholar] [CrossRef]
- Jin, S.P.; Li, Z.; Choi, E.K.; Lee, S.; Kim, Y.K.; Seo, E.Y.; Chung, J.H.; Cho, S. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J. Dermatol. Sci. 2018, 91, 175–183. [Google Scholar] [CrossRef]
- Church, D.F.; Pryor, W.A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect. 1985, 64, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.A.; Tomkins, B.; Guerin, M.R. The Chemistry of Environmental Tobacco Smoke: Composition and Measurement; CRC Press LLC: Boca Raton, FL, USA, 2000; pp. 5–14. [Google Scholar]
- Eiserich, J.; Vossen, V.; O’Neill, C.A.; Halliwell, B.; Cross, C.E.; van der Vliet, A. Molecular mechanisms of damage by excess nitrogen oxides: Nitration of tyrosine by gas-phase cigarette smoke. FEBS Lett. 1994, 353, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Woodby, B.; Prieux, R.; Valacchi, G. Involvement of 4-hydroxy-2-nonenal in pollution-induced skin damage. BioFactors 2019, 45, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Borgerding, M.; Klus, H. Analysis of complex mixtures—Cigarette smoke. Exp. Toxicol. Pathol. 2005, 57, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Laisk, A.; Kull, O.; Moldau, H. Ozone concentration in leaf intercellular air spaces is close to zero. Plant Physiol. 1989, 90, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Sarti, P.; Avigliano, L.; Görlach, A.; Brüne, B. Superoxide and nitric oxide-participation in cell communication. Cell Death Differ. 2002, 9, 1160–1162. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zheng, H.; Xiang, H.; Fan, J.; Jiang, H. The surface degradation and release of microplastics from plastic films studied by UV radiation and mechanical abrasion. Sci. Total Environ. 2022, 838, 156369. [Google Scholar] [CrossRef]
- Amato-lourenço, L.F.; Galvão, S.; De Weger, L.A.; Hiemstra, P.S. An emerging class of air pollutants: Potential effects of microplastics to respiratory human health? Sci Total Environ. 2020, 49, 141676. [Google Scholar] [CrossRef]
- Kik, K.; Bukowska, B.; Sicińska, P. Polystyrene nanoparticles: Sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ. Pollut. 2020, 262, 114297. [Google Scholar] [CrossRef]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(nano)plastics: A threat to human health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Ferrara, F.; Pecorelli, A.; Valacchi, G. Redox Regulation of Nucleotide-Binding and Oligomerization Domain-Like Receptors Inflammasome. Antioxid Redox Signal. 2023, 39, 744–770. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Prieux, R.; Woodby, B.; Valacchi, G. Inflammasome Activation in Pollution-Induced Skin Conditions. Plast. Reconstr. Surg. 2021, 147, 15S–24S. [Google Scholar] [CrossRef] [PubMed]
- Mejias, N.H.; Martinez, C.C.; Stephens, M.E.; De Rivero Vaccari, J.P. Contribution of the inflammasome to inflammaging. J. Inflamm. 2018, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Kanneganti, T.D. Inflammasome activation and assembly at a glance. J. Cell Sci. 2017, 130, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Li, A.A.; Zhang, Y.; Tong, W.L.; Chen, J.W.; Huang, S.H.; Liu, J.M.; Liu, Z.L. Identification of a Novel Pyroptosis-Related Gene Signature Indicative of Disease Prognosis and Treatment Response in Skin Cutaneous Melanoma. Int. J. Gen. Med. 2022, 15, 6145–6163. [Google Scholar] [CrossRef]
- Beer, H.D.; Contassot, E.; French, L.E. The inflammasomes in autoinflammatory diseases with skin involvement. J. Investig. Dermatol. 2014, 134, 1805–1810. [Google Scholar] [CrossRef]
- Awad, F.; Assrawi, E.; Louvrier, C.; Jumeau, C.; Giurgea, I.; Amselem, S.; Karabina, S.A. Photoaging and skin cancer: Is the inflammasome the missing link? Mech. Ageing Dev. 2018, 172, 131–137. [Google Scholar] [CrossRef]
- Nakamichi, S.; Origuchi, T.; Fukui, S.; Yoda, A.; Matsubara, H.; Nagaura, Y.; Nishikomori, R.; Abe, K.; Migita, K.; Sakamoto, N.; et al. A rare case of cryopyrin-associated periodic syndrome in an elderly woman with nlrp3 and mefv mutations. Intern. Med. 2019, 58, 1017–1022. [Google Scholar] [CrossRef]
- Zhong, F.L.; Mamaï, O.; Sborgi, L.; Boussofara, L.; Hopkins, R.; Robinson, K.; Szeverényi, I.; Takeichi, T.; Balaji, R.; Lau, A.; et al. Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation. Cell 2016, 167, 187–202.e17. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, T.; Yu, Z.; Yu, Q.; Wang, Y.; Hu, J.; Shi, J.; Yang, G. The functions and roles of sestrins in regulating human diseases. Cell. Mol. Biol. Lett. 2022, 27, 2. [Google Scholar] [CrossRef] [PubMed]
- Vande Walle, L.; Lamkanfi, M. Pyroptosis. Curr. Biol. 2016, 26, R568–R572. [Google Scholar] [CrossRef] [PubMed]
- Sborgi, L.; Rühl, S.; Mulvihill, E.; Pipercevic, J.; Heilig, R.; Farady, C.J.; Müller, D.J.; Broz, P.; Hiller, S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016, 35, 1766–1778. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Jagdeo, J.; Kurtti, A.; Hernandez, S.; Akers, N.; Peterson, S. Novel Vitamin C and E and Green Tea Polyphenols Combination Serum Improves Photoaged Facial Skin. J. Drugs Dermatol. 2021, 20, 996–1003. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Lim, D.; Lee, Y.K.; Kim, J.H. Effects of indoor air pollutants on atopic dermatitis. Int. J. Environ. Res. Public Health 2016, 13, 1220. [Google Scholar] [CrossRef]
- Kimata, H. Exposure to road traffic enhances allergic skin wheal responses and increases plasma neuropeptides and neurotrophins in patients with atopic eczema/dermatitis syndrome. Int. J. Hyg. Environ. Health 2004, 207, 45–49. [Google Scholar] [CrossRef]
- Ding, A.; Yang, Y.; Zhao, Z.; Hüls, A.; Vierkötter, A.; Yuan, Z.; Cai, J.; Zhang, J.; Gao, W.; Li, J.; et al. Indoor PM2.5 exposure affects skin aging manifestation in a Chinese population. Sci. Rep. 2017, 7, 15329. [Google Scholar] [CrossRef]
- Poli, G.; Schaur, R.J.; Siems, W.A.; Leonarduzzi, G. 4-Hydroxynonenal: A Membrane Lipid Oxidation Product of Medicinal Interest. Med. Res. Rev. 2008, 28, 569–631. [Google Scholar] [CrossRef]
- Poli, G.; Chiarpotto, E.; Biasi, F.; Pavia, R.; Albano, E.; Dianzani, M.U. Enzymatic impairment induced by biological aldehydes in intact rat liver cells. Res. Commun. Chem. Pathol. Pharmacol. 1982, 38, 71–76. [Google Scholar] [PubMed]
- Uchida, K. 4-Hydroxy-2-nonenal: A product and mediator of oxidative stress. Prog. Lipid Res. 2003, 42, 318–343. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.P.; Jung, T.; Grune, T.; Siems, W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic. Biol. Med. 2017, 111, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Codreanu, S.G.; Zhang, B.; Sobecki, S.M.; Billheimer, D.D.; Liebler, D.C. Global analysis of protein damage by the lipid electrophile 4-hydroxy-2-nonenal. Mol. Cell. Proteom. 2009, 8, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Delsignore, M.E. Protein damage and degradation by oxygen radicals. III. Modification of secondary and tertiary structure. J. Biol. Chem. 1987, 262, 9908–9913. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Delsignore, M.E.; Lin, S.W. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J. Biol. Chem. 1987, 262, 9902–9907. [Google Scholar] [CrossRef]
- Zeng, J.; Lu, J. Mechanisms of action involved in ozone-therapy in skin diseases. Int. Immunopharmacol. 2018, 56, 235–241. [Google Scholar] [CrossRef]
- Valacchi, G.; Muresan, X.M.; Sticozzi, C.; Belmonte, G.; Pecorelli, A.; Cervellati, F.; Demaude, J.; Krol, Y.; Oresajo, C. Ozone-induced damage in 3D-Skin Model is prevented by topical vitamin C and vitamin E compound mixtures application. J. Dermatol. Sci. 2016, 82, 209–212. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhu, X.M.; Yao, Y.M. Sestrin2: Its Potential Role and Regulatory Mechanism in Host Immune Response in Diseases. Front. Immunol. 2019, 10, 2797. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, P.; Guo, S.; Yi, X.; Wang, H.; Yang, Y.; Liu, L.; Shi, Q.; Gao, T.; Li, C. Metastatic Melanoma Cells Rely on Sestrin2 to Acquire Anoikis Resistance via Detoxifying Intracellular ROS. J. Investig. Dermatol. 2020, 140, 666–675.e2. [Google Scholar] [CrossRef]
- Edamitsu, T.; Taguchi, K.; Okuyama, R.; Yamamoto, M. AHR and NRF2 in Skin Homeostasis and Atopic Dermatitis. Antioxidants 2022, 11, 227. [Google Scholar] [CrossRef]
- Ala, M.; Eftekhar, S.P. Target Sestrin2 to Rescue the Damaged Organ: Mechanistic Insight into Its Function. Oxid. Med. Cell. Longev. 2021, 2021, 8790369. [Google Scholar] [CrossRef]
- Shin, B.Y.; Jin, S.H.; Cho, I.J.; Ki, S.H. Nrf2-ARE pathway regulates induction of Sestrin-2 expression. Free Radic. Biol. Med. 2012, 53, 834–841. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Zastrow, L.; Meinke, M.C.; Albrecht, S.; Patzelt, A.; Lademann, J. From UV protection to protection in the whole spectral range of the solar radiation: New aspects of sunscreen development. Adv. Exp. Med. Biol. 2017, 996, 311–318. [Google Scholar]
- Herath, H.M.U.L.; Piao, M.J.; Kang, K.A.; Zhen, A.X.; Fernando, P.D.S.M.; Kang, H.K.; Yi, J.M.; Hyun, J.W. Hesperidin Exhibits Protective Effects against PM2.5-Mediated Mitochondrial Damage, Cell Cycle Arrest, and Cellular Senescence in Human HaCaT Keratinocytes. Molecules 2022, 27, 4800. [Google Scholar] [CrossRef]
- Valacchi, G.; Pagnin, E.; Okamoto, T.; Corbacho, A.M.; Olano, E.; Davis, P.A.; Van Der Vliet, A.; Packer, L.; Cross, C.E. Induction of stress proteins and MMP-9 by 0.8 ppm of ozone in murine skin. Biochem. Biophys. Res. Commun. 2003, 305, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Riihilä, P.; Nissinen, L.; Kähäri, V.M. Matrix metalloproteinases in keratinocyte carcinomas. Exp. Dermatol. 2021, 30, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.B.; Houben, R.; Bröcker, E.; Becker, J.C. Role of matrix metalloproteinases in melanoma cell invasion. Biochimie 2005, 87, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Grandemange, S.; Sanchez, E.; Louis-Plence, P.; Rittore, C.; Reed, J.; Touitou, I.; Geneviève, D. NLRP1 mutations cause autoinflammatory diseases in human. Pediatr. Rheumatol. 2015, 13, O22. [Google Scholar] [CrossRef]
- Zwicker, S.; Hattinger, E.; Bureik, D.; Batycka-Baran, A.; Schmidt, A.; Gerber, P.A.; Rothenfusser, S.; Gilliet, M.; Ruzicka, T.; Wolf, R. Th17 micro-milieu regulates NLRP1-dependent caspase-5 activity in skin autoinflammation. PLoS ONE 2017, 12, e0175153. [Google Scholar] [CrossRef]
- Ciążyńska, M.; Bednarski, I.A.; Wódz, K.; Narbutt, J.; Lesiak, A. NLRP1 and NLRP3 inflammasomes as a new approach to skin carcinogenesis (Review). Oncol. Lett. 2020, 19, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Burian, M.; Yazdi, A.S. NLRP1 Is the Key Inflammasome in Primary Human Keratinocytes. J. Investig. Dermatol. 2018, 138, 2507–2510. [Google Scholar] [CrossRef]
- Rajendiran, K.S.; Rajappa, M.; Chandrashekar, L.; Thappa, D.M. Association of Nod-like receptor protein-1 (rs2670660) and Toll-like receptor-4 (rs4986790) with non-segmental vitiligo: A case–control study in South Indian population. Int. J. Immunogenet. 2019, 46, 321–330. [Google Scholar] [CrossRef]
- Yu, C.H.; Moecking, J.; Geyer, M.; Masters, S.L. Mechanisms of NLRP1-Mediated Autoinflammatory Disease in Humans and Mice. J. Mol. Biol. 2018, 430, 142–152. [Google Scholar] [CrossRef]
- Ekman, A.K.; Verma, D.; Fredrikson, M.; Bivik, C.; Enerbäck, C. Genetic variations of NLRP1: Susceptibility in psoriasis. Br. J. Dermatol. 2014, 171, 1517–1520. [Google Scholar] [CrossRef]
- Jang, H.Y.; Koo, J.H.; Lee, S.M.; Park, B.H. Atopic dermatitis-like skin lesions are suppressed in fat-1 transgenic mice through the inhibition of inflammasomes. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Niebuhr, M.; Baumert, K.; Heratizadeh, A.; Satzger, I.; Werfel, T. Impaired NLRP3 inflammasome expression and function in atopic dermatitis due to Th2 milieu. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.A.; Huang, Y.T.; Hsiao, P.F.; Chiu, L.Y.; Chern, S.R.; Wu, N.L. Critical roles of irradiance in the regulation of UVB-induced inflammasome activation and skin inflammation in human skin keratinocytes. J. Photochem. Photobiol. B Biol. 2022, 226, 112373. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Cordone, V.; Pecorelli, A.; Benedusi, M.; Pambianchi, E.; Guiotto, A.; Vallese, A.; Cervellati, F.; Valacchi, G. Ubiquitination as a key regulatory mechanism for O 3 -induced cutaneous redox inflammasome activation. Redox Biol. 2022, 56, 102440. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.J.; Tang, S.C.; Liao, P.Y.; Ge, J.S.; Hsiao, Y.P.; Yang, J.H. Photoprotective Potential of Glycolic Acid by Reducing NLRC4 and AIM2 Inflammasome Complex Proteins in UVB Radiation-Induced Normal Human Epidermal Keratinocytes and Mice. DNA Cell Biol. 2017, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Fenini, G.; Karakaya, T.; Hennig, P.; Di Filippo, M.; Beer, H.D. The NLRP1 inflammasome in human skin and beyond. Int. J. Mol. Sci. 2020, 21, 4788. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.K.; Vollmer, S.; Perez-Oliva, A.B. Posttranslational Regulation of Inflammasomes, Its Potential as Biomarkers and in the Identification of Novel Drugs Targets. Front. Cell Dev. Biol. 2022, 10, 887533. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Jerome, J.A.; Gregory, A.D.; Mallampalli, R.K. Cigarette smoke destabilizes NLRP3 protein by promoting its ubiquitination. Respir. Res. 2017, 18, 2. [Google Scholar] [CrossRef]
- Poyet, J.L.; Srinivasula, S.M.; Tnani, M.; Razmara, M.; Fernandes-Alnemri, T.; Alnemri, E.S. Identification of Ipaf, a Human Caspase-1-activating Protein Related to Apaf-1. J. Biol. Chem. 2001, 276, 28309–28313. [Google Scholar] [CrossRef] [PubMed]
- Faustin, B.; Lartigue, L.; Bruey, J.M.; Luciano, F.; Sergienko, E.; Bailly-Maitre, B.; Volkmann, N.; Hanein, D.; Rouiller, I.; Reed, J.C. Reconstituted NALP1 Inflammasome Reveals Two-Step Mechanism of Caspase-1 Activation. Mol. Cell 2007, 25, 713–724. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Boveresses, C.; Epalinges, C. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of proIL-b. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Levy, M.; Thaiss, C.A.; Zeevi, D.; Dohnalová, L.; Zilberman-Schapira, G.; Mahdi, J.A.; David, E.; Savidor, A.; Korem, T.; Herzig, Y.; et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 2015, 163, 1428–1443. [Google Scholar] [CrossRef]
- Birchenough, G.M.; Nyström, E.E.; Johansson, M.E.; Hansson, G.C. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science 2016, 352, 1535–1542. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Nagaoka, I.; Okumura, K.; Ogawa, H. The Human β-Defensins (-1, -2, -3, -4) and Cathelicidin LL-37 Induce IL-18 Secretion through p38 and ERK MAPK Activation in Primary Human Keratinocytes. J. Immunol. 2005, 175, 1776–1784. [Google Scholar] [CrossRef]
- Van der Does, A.M.; Beekhuizen, H.; Ravensbergen, B.; Vos, T.; Ottenhoff, T.H.M.; van Dissel, J.T.; Drijfhout, J.W.; Hiemstra, P.S.; Nibbering, P.H. LL-37 Directs Macrophage Differentiation toward Macrophages with a Proinflammatory Signature. J. Immunol. 2010, 185, 1442–1449. [Google Scholar] [CrossRef]
- Ellis, S.R.; Nguyen, M.; Vaughn, A.R.; Notay, M.; Burney, W.A.; Sandhu, S.; Sivamani, R.K. The skin and gut microbiome and its role in common dermatologic conditions. Microorganisms 2019, 7, 550. [Google Scholar] [CrossRef]
- Clausen, M.L.; Slotved, H.C.; Krogfelt, K.A.; Agner, T. Measurements of AMPs in stratum corneum of atopic dermatitis and healthy skin-tape stripping technique. Sci. Rep. 2018, 8, 1666. [Google Scholar] [CrossRef] [PubMed]
- Ozlu, E.; Karadag, A.S.; Ozkanli, S.; Oguztuzun, S.; Akbulak, O.; Uzuncakmak, T.K.; Demirkan, S.; Akdeniz, N. The investigation of antimicrobial peptides expression and its related interaction with methotrexate treatment in patients with psoriasis vulgaris. Cutan. Ocul. Toxicol. 2017, 36, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Uzuncakmak, T.K.; Karadag, A.S.; Ozkanli, S.; Akbulak, O.; Ozlu, E.; Akdeniz, N.; Oguztuzun, S. Alteration of tissue expression of human beta defensin-1 and human beta defensin-2 in psoriasis vulgaris following phototherapy. Biotech. Histochem. 2020, 95, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Woodby, B.; Pambianchi, E.; Ferrara, F.; Therrien, J.P.; Pecorelli, A.; Messano, N.; Lila, M.A.; Valacchi, G. Cutaneous antimicrobial peptides: New “actors” in pollution related inflammatory conditions. Redox Biol. 2021, 41, 101952. [Google Scholar] [CrossRef]
- Ferrara, F.; Woodby, B.; Pecorelli, A.; Schiavone, M.L.; Pambianchi, E.; Messano, N.; Therrien, J.P.; Choudhary, H.; Valacchi, G. Additive effect of combined pollutants to UV induced skin OxInflammation damage. Evaluating the protective topical application of a cosmeceutical mixture formulation. Redox Biol. 2020, 34, 101481. [Google Scholar] [CrossRef]
- Pambianchi, E.; Ferrara, F.; Pecorelli, A.; Benedusi, M.; Choudhary, H.; Therrien, J.P.; Valacchi, G. Deferoxamine treatment improves antioxidant cosmeceutical formulation protection against cutaneous diesel engine exhaust exposure. Antioxidants 2021, 10, 1928. [Google Scholar] [CrossRef]





| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| NLRP1 | ACCCTCTTAACTCCGGGACA | GAGTGCGCTTTATTGGCGAG |
| NLRP3 | CGGGGCCTCTTTTCAGTTCT | CCCCAACCACAATCTCCGAA |
| NLRP6 | CCTGTGAAGGAATCACCTCTCT | GTCCATGGGGTCTCTTCCTCC |
| ASC | ATGCGCTGGAGAACCTGA | TCTCCAGGTAGAAGCTGACCA |
| IL-1β | CACGATGCACCTGTACGATCA | GTTGCTCCATATCCTGTCCCT |
| GAPDH | TCGGAGTCAACGGATTTGGT | TTCCCGTTCTCAGCCTTGAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivarsson, J.; Ferrara, F.; Vallese, A.; Guiotto, A.; Colella, S.; Pecorelli, A.; Valacchi, G. Comparison of Pollutant Effects on Cutaneous Inflammasomes Activation. Int. J. Mol. Sci. 2023, 24, 16674. https://doi.org/10.3390/ijms242316674
Ivarsson J, Ferrara F, Vallese A, Guiotto A, Colella S, Pecorelli A, Valacchi G. Comparison of Pollutant Effects on Cutaneous Inflammasomes Activation. International Journal of Molecular Sciences. 2023; 24(23):16674. https://doi.org/10.3390/ijms242316674
Chicago/Turabian StyleIvarsson, John, Francesca Ferrara, Andrea Vallese, Anna Guiotto, Sante Colella, Alessandra Pecorelli, and Giuseppe Valacchi. 2023. "Comparison of Pollutant Effects on Cutaneous Inflammasomes Activation" International Journal of Molecular Sciences 24, no. 23: 16674. https://doi.org/10.3390/ijms242316674