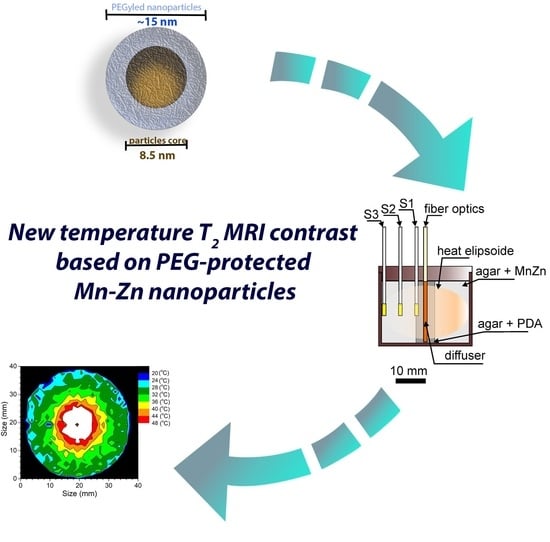
Aqueous Dispersion of Manganese–Zinc Ferrite Nanoparticles Protected by PEG as a T2 MRI Temperature Contrast Agent
Abstract
:1. Introduction
2. Results
2.1. Chemical and Morphological Characterization
2.2. TG/DTG Analysis
2.3. Magnetic Properties
2.4. Nuclear Relaxation
2.5. Magnetic Resonance Imaging
2.6. Diffusion Measurements
2.7. Biological Study
3. Discussion
3.1. Spin–Spin Relaxation in Motional Averaging Regime
3.2. Spatial–Thermal Resolution of the Temperature Determination
3.3. Comparison of MnZn with CuZn
3.4. Limitations of Thermometry Using MRI Temperature-Sensitive Contrast Agent
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PEG Coated Nanoparticles
4.3. Morphology Characterization Methods
4.4. Thermogravimetric Analysis (TGA)
4.5. Magnetization Measurements
4.6. Nuclear Relaxation
4.7. Diffusion Measurements
4.8. Magnetic Resonance Imaging Protocol
4.9. Biological Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Magnetic Resonance Imaging Setup

References
- Curie, P. Lois expérimentales du magnétisme. Propriétés magnétiques des corps à diverses températures. Ann. Chim. Phys. 1895, 5, 289. [Google Scholar]
- Settecase, F.; Sussman, M.S.; Roberts, T.P.L. A new temperature-sensitive contrast mechanism for MRI: Curie temperature transition-based imaging. Contrast Media Mol. Imaging 2007, 2, 50. [Google Scholar] [CrossRef]
- Zhong, J.; Schilling, M.; Ludwig, F. Magnetic nanoparticle temperature imaging with a scanning magnetic particle spectrometer. Meas. Technol. 2018, 29, 115903. [Google Scholar] [CrossRef]
- Woodrum, D.A.; Kawashima, A.; Gorny, K.R.; Mynderse, L.A. Targeted prostate biopsy and MR-guided therapy for prostate cancer. Abdom. Radiol. 2016, 41, 877. [Google Scholar] [CrossRef] [PubMed]
- Rieke, V.; Pauly, K.B. MR Thermometry. J. Magn. Reson. Imaging 2008, 27, 376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Sun, Z.; Ng, C.K. Image-guided thermal ablation with MR-based thermometry. Quant. Imaging Med. Surg. 2017, 7, 356. [Google Scholar] [CrossRef]
- Dzwonczyk, R.; Fujii, J.T.; Simonetti, O.; Nieves-Ramos, R.; Bergese, S.D. Electrical noise in the intraoperative magnetic resonance imaging setting. Anesth. Analg. 2009, 108, 181. [Google Scholar] [CrossRef]
- Odéen, H.; Parker, D.L. Magnetic resonance thermometry and its biological–physical principles and practical considerations. Prog. NMR Spectrosc. 2019, 110, 34. [Google Scholar] [CrossRef]
- Hofstetter, L.W.; Yeo, D.T.B.W.; Dixon, T.; Marinelli, L.; Foo, T.K. Referenced MR thermometry using three-echo phase-based fat water separation method. Magn. Reson. Imaging 2018, 49, 86. [Google Scholar] [CrossRef]
- Hankiewicz, J.H.; Celinski, Z.; Stupic, K.F.; Anderson, N.R.; Camley, R.E. Ferromagnetic particles as magnetic resonance imaging temperature sensors. Nat. Commun. 2016, 7, 12415. [Google Scholar] [CrossRef]
- Alghamdi, N.A.; Hankiewicz, J.H.; Anderson, N.R.; Stupic, K.F.; Camley, R.E.; Przybylski, M.; Żukrowski, J.; Celinski, Z. Development of ferrite-based temperature sensors for magnetic resonance imaging: A study of Cu1−xZnxFe2O4. Phys. Rev. Appl. 2018, 9, 54030. [Google Scholar] [CrossRef]
- Lachowicz, D.; Stroud, J.; Hankiewicz, J.H.; Gassen, R.; Kmita, A.; Stepień, J.; Celinski, Z.; Sikora, M.; Zukrowski, J.; Gajewska, M.; et al. One-step preparation of highly stable copper–zinc ferrite nanoparticles in water suitable for MRI thermometry. Chem. Mater. 2022, 34, 4001. [Google Scholar] [CrossRef] [PubMed]
- Antarnusa, G.; Suharyadi, E. A synthesis of polyethylene glycol (PEG)-coated magnetite Fe3O4 nanoparticles and their characteristics for enhancement of biosensor. Mater. Res. Express 2020, 7, 056103. [Google Scholar] [CrossRef]
- Tovar-Spinoza, Z.; Carter, D.; Ferrone, D.; Eksioglu, Y.; Huckins, S. The use of MRI-guided laser-induced thermal ablation for epilepsy. Childs Nerv. Syst. 2013, 29, 2089. [Google Scholar] [CrossRef]
- Smit, J.; Wijn, H.P.J. Ferrites; Wiley: New York, NY, USA, 1959. [Google Scholar]
- Pan, D.; Schmieder, A.H.; Wickline, S.A.; Lanza, G.M. Manganese-based MRI contrast agents: Past, present, and future. Tetrahedron 2011, 67, 8431. [Google Scholar] [CrossRef] [PubMed]
- Gale, E.M.; Wey, H.-Y.; Ramsay, I.; Yen, Y.-F.; Sosnovik, D.E.; Caravan, P. A Manganese-based alternative to gadolinium: Contrast-enhanced MR angiography, excretion, pharmacokinetics, and metabolism. Radiology 2018, 286, 865. [Google Scholar] [CrossRef] [PubMed]
- Kálmán, F.K.; Nagy, V.; Váradi, B.; Garda, Z.; Molnár, E.; Trencsényi, G.; Kiss, J.; Même, S.; Même, W.; Tóth, É.; et al. Mn(II)-based MRI contrast agent candidate for vascular imaging. J. Med. Chem. 2020, 63, 6057. [Google Scholar] [CrossRef]
- Saar, G.; Koretsky, A.P. Manganese enhanced MRI for use in studying neurodegenerative diseases. Front. Neural. Circuits 2019, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Rezlescu, N.; Cuciureanu, E. Cation distribution and curie temperature in some ferrites containing copper and manganese. Phys. Status Solidi 1970, 3, 873. [Google Scholar] [CrossRef]
- Arulmurugan, R.; Vaidyanathan, G.; Sendhilnathan, S.; Jeyadevan, B. Mn–Zn ferrite nanoparticles for ferrofluid preparation: Study on thermal—Magnetic properties. J. Magn. Magn. Mater. 2006, 298, 83. [Google Scholar] [CrossRef]
- Mirshekari, G.R.; Daee, S.S.; Mohseni, H.; Torkian, S.; Ghasemi, M.; Ameriannejad, M.; Hoseinizade, M.; Pirnia, M.; Pourjafar, D.; Pourmahdavi, M.; et al. Structure and magnetic properties of Mn-Zn ferrite synthesized by glycine-nitrate auto-combustion process. Adv. Mat. Res. 2011, 349, 520. [Google Scholar] [CrossRef]
- Thakur, P.; Taneja, S.; Sindhu, D.; Lüders, U.; Sharma, A.; Ravelo, B.; Thakur, A. Manganese zinc ferrites: A short review on synthesis and characterization. J. Supercond. Nov. Magn. 2020, 33, 1569. [Google Scholar] [CrossRef]
- Bram, S.; Gordon, M.N.; Carbonell, M.A.; Pink, M.; Stein, B.D.; Morgan, D.G.; Aguilà, D.; Aromí, G.; Skrabalak, S.E.; Losovyj, Y.; et al. Zn2+ ion surface enrichment in doped iron oxide nanoparticles leads to charge carrier density enhancement. ACS Omega 2018, 3, 16328. [Google Scholar] [CrossRef]
- Szczerba, W.; Żukrowski, J.; Przybylski, M.; Sikora, M.; Safonova, O.; Shmeliov, A.; Nicolos, V.; Schneide, M.; Granath, T.; Oppmann, M.; et al. Pushing up the magnetisation values for iron oxide nanoparticles: Via zinc doping: X-ray studies on the particle’s sub-nano structure of different synthesis routes. Phys. Chem. Chem. Phys. 2016, 18, 25221. [Google Scholar] [CrossRef]
- Lachowicz, D.; Wirecka, R.; Górka-Kumik, W.; Marzec, M.M.; Gajewska, M.; Kmita, A.; Żukrowski, J.; Sikora, M.; Zapotoczny, S.; Bernasik, A. Gradient of zinc content in the core-shell zinc ferrites nanoparticles–precise study on composition and magnetic properties. Phys. Chem. Chem. Phys. 2019, 21, 23473. [Google Scholar] [CrossRef]
- Parekh, K.; Upadhyay, R.V.; Belova, L.; Rao, K.V. Ternary monodispersed Mn0.5Zn0.5Fe2O4 ferrite nanoparticles: Preparation and magnetic characterization. Nanotechnology 2006, 17, 5970. [Google Scholar] [CrossRef]
- Petrescu, L.G.; Petrescu, M.C.; Ioniță, V.; Cazacu, E.; Constantinescu, C.D. Magnetic properties of manganese-zinc soft ferrite ceramic for high frequency applications. Materials 2019, 12, 3173. [Google Scholar] [CrossRef]
- Singh, R.; Thirupathi, G. Manganese-zinc spinel ferrite nanoparticles and ferrofluids. Magnetic Spinels—Synthesis, Properties and Applications; Intechopen: London, UK, 2017. [Google Scholar] [CrossRef]
- Pabisch, S.; Feichtenschlager, B.; Kickelbick, G.; Peterlik, H. Effect of interparticle interactions on size determination of zirconia and silica based systems—A comparison of SAXS, DLS, BET, XRD and TEM. Chem. Phys. Lett. 2012, 10, 91. [Google Scholar] [CrossRef]
- Cherecheş, E.I.; Prado, J.I.; Chereches, M.; Minea, A.A.; Lugo, L. Experimental study on thermophysical properties of alumina nanoparticle enhanced ionic liquids. J. Mol. Liq. 2019, 291, 111332. [Google Scholar] [CrossRef]
- Xiaosheng, D.; Zhou, M.; Deng, S.; Zongliang, D.; Xu, C.; Haibo, W. Poly(ethylene glycol)-grafted nanofibrillated cellulose/graphene hybrid aerogels supported phase change composites with superior energy storage capacity and solar-thermal conversion efficiency. Cellulose 2020, 27, 4679. [Google Scholar] [CrossRef]
- Guillaud, C. The properties of manganese–zinc ferrites and the physical processes governing them. Proc. Inst. Electr. Eng. 1957, 104, 165–178. [Google Scholar] [CrossRef]
- Zapata, A.; Herrera, G. Effect of zinc concentration on the microstructure and relaxation frequency of Mn–Zn ferrites synthesized by solid state reaction. Ceram. Int. 2013, 39, 7853–7860. [Google Scholar] [CrossRef]
- Mirowitz, S.A. MR imaging artifacts. Challenges and solutions. Magn. Reson. Imaging Clin. North Am. 1999, 7, 717. [Google Scholar] [CrossRef]
- Krupa, K.; Bekiesińska-Figatowska, M. Artifacts in magnetic resonance imaging. Pol. J. Radiol. 2015, 80, 93. [Google Scholar] [CrossRef] [PubMed]
- Sack, I.; Gedat, E.; Bernarding, J.; Buntkowsky, G.; Braun, J. Magnetic resonance elastography and diffusion-weighted imaging of the sol/gel phase transition in agarose. J. Magn. Reson. 2004, 166, 252–261. [Google Scholar] [CrossRef]
- Davies, E.; Huang, Y.; Harper, J.B.; Hook, J.M.; Thomas, D.S.; Burgar, I.M.; Lillford, P.J. Dynamics of water in agar gels studied using low and high resolution 1H NMR spectroscopy. Int. J. Food Sci. Technol. 2010, 45, 2502. [Google Scholar] [CrossRef]
- Huang, Y.; Davies, E.; Lillford, P. Effect of solutes and matrix structure on water mobility in glycerol-agar-water gel systems: A nuclear magnetic resonance approach. J. Agric. Food Chem. 2011, 59, 4078. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Hankiewicz, J.H.; Celinski, Z.; Camley, R.E. Measurement of sub-zero temperatures in MRI using T1 temperature sensitive soft silicone materials: Applications for MRI-guided cryosurgery. Med. Phys. 2021, 48, 6844. [Google Scholar] [CrossRef]
- Yablonskiy, D.A.; Haacke, E.M. Theory of NMR signal behavior in inhomogeneous tissues: The static dephasing regime. Magn. Reson. Med. 1994, 32, 749. [Google Scholar] [CrossRef]
- Vuong, Q.L.; Berret, J.-F.; Fresnais, J.; Gossuin, Y.; Sandre, O.A. Universal scaling law to predict the efficiency of magnetic nanoparticles as MRI T2-contrast agents. Adv. Healthc. Mater. 2012, 1, 502. [Google Scholar] [CrossRef]
- Carroll, M.R.J.; Woodward, R.C.; House, M.J.; Teoh, W.Y.; Amal, R.; Hanley, T.L.; St Pierre, T.G. Experimental validation of proton transverse relaxivity models for superparamagnetic nanoparticle MRI contrast agents. Nanotechnology 2010, 21, 035103. [Google Scholar] [CrossRef] [PubMed]
- Brero, F.; Arosio, P.; Albino, M.; Cicolari, D.; Porru, M.; Basini, M.; Mariani, M.; Innocenti, C.; Sangregorio, C.; Orsini, F.; et al. 1H-NMR Relaxation of ferrite core-shell nanoparticles: Evaluation of the coating effect. Nanomater. 2023, 13, 804. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.H.; Kellar, K.E. Theory of 1/T1 and 1/T2 NMRD profiles of solutions of magnetic nanoparticles. Magn. Reson. Med. 1995, 34, 227. [Google Scholar] [CrossRef]
- Hankiewicz, J.; Stroud, J. Unpublished results.
- Chen, D.X.; Taboada, E.; Roig, A. Experimental study on T2 relaxation time of protons in water suspensions of iron-oxide nanoparticles: Cases of composite nanospheres. J. Magn. Magn. Mater. 2011, 323, 2487. [Google Scholar] [CrossRef]
- Deka, K.; Guleria, A.; Kumar, D.; Biswas, J.; Lodha, S.; Kaushik, S.D.; Choudhary, S.A.; Dasgupta, S.; Deb, P. Janus nanoparticles for contrast enhancement of T1–T2 dual mode magnetic resonance imaging. Dalton Trans. 2019, 48, 1075. [Google Scholar] [CrossRef]
- Zhou, Z.; Bai, R.; Munasinghe, J.; Shen, Z.; Nie, L.; Chen, X. T1-T2 Dual-modal magnetic resonance imaging: From molecular basis to contrast agents. ACS Nano 2017, 11, 5227. [Google Scholar] [CrossRef]
- Nobles, J.E.; Hao, Y.; Goldman, S.; Stroud, S.; Stupic, K.; Hankiewicz, J.H.; Celinski, Z. Employing gadolinium micro-disks as temperature probes for magnetic resonance imaging. J. Magn. Magn. Mater. 2022, 562, 169849. [Google Scholar] [CrossRef]
- Stroud, J.; Hao, Y.; Read, T.S.; Hankiewicz, J.H.; Bilski, P.; Klodowski, K.; Brown, J.M.; Rogers, K.; Stoll, J.; Camley, R.E.; et al. Magnetic particle based MRI thermometry at 0.2 T and 3 T. Magn. Res. Imaging 2023, 100, 43–54. [Google Scholar] [CrossRef]
- Stoll, J.; Lachowicz, D.; Kmita, A.; Gajewska, M.; Sikora, M.; Berent, K.; Przybylski, M.; Russek, S.E.; Celinski, Z.; Hankiewicz, J.H. Synthesis of manganese zinc ferrite nanoparticles in medical-grade silicone for MRI applications. Int. J. Mol. Sci. 2023, 24, 5685. [Google Scholar] [CrossRef]
- Available online: http://www.cis.kit.ac.jp/~kiki/UAmanual.pdf (accessed on 1 September 2023).
- Boss, M.A.; Dienstfrey, A.M.; Gimbutas, Z.; Keenan, K.E.; Kos, A.B.; Splett, J.D.; Stupic, K.F.; Russek, S.E. Magnetic resonance imaging biomarker calibration service: Proton spin relaxation times. NIST Spec. Publ. 2018, 250-97, 1–29. [Google Scholar] [CrossRef]
- Berger, S.; Braun, S. 200 and More NMR Experiments; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004. [Google Scholar]
- Dietrich, O.; Biffar, A.; Baur-Melnyk, A.; Reiser, M.F. Technical aspects of: MR diffusion imaging of the body. Eur. J. Radiol. 2010, 76, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chen, X.; Yang, P.; Liang, G.; Yang, Y.; Gu, Z.; Li, Y. Regulating the absorption spectrum of polydopamine. Sci. Adv. 2020, 6, eabb4696. [Google Scholar] [CrossRef]
- Mrowczynski, R. Polydopamine-based multifunctional (nano)materials for cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 7541. [Google Scholar] [CrossRef] [PubMed]









| ADC (10−3 mm2s−1) | ||||
|---|---|---|---|---|
| T (°C) | DIW | 0.5% Agar | 1% Agar | 2% Agar |
| 5 | 1.34 | 1.33 | 1.29 | 1.27 |
| 20 | 2.03 | 2.01 | 1.97 | 1.92 |
| 37 | 3.00 | 2.97 | 2.91 | 2.83 |
| 50 | 3.90 | 3.86 | 3.79 | 3.70 |
| Composition | Mn0.48Zn0.46Fe2.06O4 | Cu0.08Zn0.54Fe2.38O4 |
|---|---|---|
| Size: core/shell (nm) | 8.5/15 | 6/30 |
| Mm at 40 °C (Am2kg−1) | 18.4 | 11.8 |
| Mm drop in range 5–50 °C (%) | 14.4 | 7.3 |
| Concentration of Fe (mM/mg/mL) | 0.30/0.017 | 2.02/0.128 |
| T1 change in range 5–50 °C (%) | −16.0 | −14.0 |
| T2 change in range 5–50 °C | 137.0 | 92.1 |
| r1 at 40 °C (s−1 mM−1) | 22.5 | 0.80 |
| r2 at 40 °C (s−1 mM−1) | 494.5 | 77.4 |
| Imaging time (s) | 16 | 256 |
| Thermal gradient produced by: | heating by laser | cooling by Teflon block |
| Type of thermal gradient | radial | linear |
| Thermal/spatial resolution (°C/mm) | 3.2/2.9 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lachowicz, D.; Kmita, A.; Gajewska, M.; Trynkiewicz, E.; Przybylski, M.; Russek, S.E.; Stupic, K.F.; Woodrum, D.A.; Gorny, K.R.; Celinski, Z.J.; et al. Aqueous Dispersion of Manganese–Zinc Ferrite Nanoparticles Protected by PEG as a T2 MRI Temperature Contrast Agent. Int. J. Mol. Sci. 2023, 24, 16458. https://doi.org/10.3390/ijms242216458
Lachowicz D, Kmita A, Gajewska M, Trynkiewicz E, Przybylski M, Russek SE, Stupic KF, Woodrum DA, Gorny KR, Celinski ZJ, et al. Aqueous Dispersion of Manganese–Zinc Ferrite Nanoparticles Protected by PEG as a T2 MRI Temperature Contrast Agent. International Journal of Molecular Sciences. 2023; 24(22):16458. https://doi.org/10.3390/ijms242216458
Chicago/Turabian StyleLachowicz, Dorota, Angelika Kmita, Marta Gajewska, Elżbieta Trynkiewicz, Marek Przybylski, Stephen E. Russek, Karl F. Stupic, David A. Woodrum, Krzysztof R. Gorny, Zbigniew J. Celinski, and et al. 2023. "Aqueous Dispersion of Manganese–Zinc Ferrite Nanoparticles Protected by PEG as a T2 MRI Temperature Contrast Agent" International Journal of Molecular Sciences 24, no. 22: 16458. https://doi.org/10.3390/ijms242216458