Natural Chalcones for the Management of Obesity Disease
Abstract
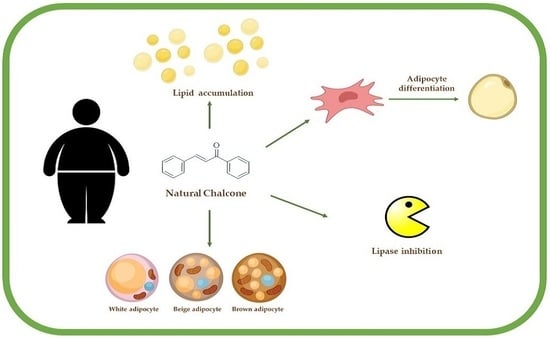
:1. Introduction
2. In Vitro Evidence
2.1. Cardamonin
2.2. Licochalcone A
2.3. Butein
2.4. Panduratin A
2.5. Isoliquiritigenin
2.6. Xanthohumol
2.7. Others
3. In Vivo Studies
3.1. Cardamonin Derivatives
3.2. Butein
3.3. Licochalcone A
3.4. Licochalcone E
3.5. Panduratin A
3.6. Isoliquiritigenin
3.7. Xanthohumol
4. Materials and Methods
Search Strategy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, K.; Chiu, H.F.; Wang, C.K. Extensive Review of Popular Functional Foods and Nutraceuticals against Obesity and Its Related Complications with a Special Focus on Randomized Clinical Trials. Food Funct. 2019, 10, 2313–2329. [Google Scholar] [CrossRef] [PubMed]
- WHO. Consideration of the Evidence on Childhood Obesity for the Commission on Ending Childhood Obesity: Report of the Ad Hoc Working Group on Science and Evidence for Ending Childhood Obesity; World Health Organization: Geneva, Switzerland, 2016; Volume 219. [Google Scholar]
- Ricci, S.; Pinto, F.; Auletta, A.; Giordano, A.; Giovane, A.; Settembre, G.; Boccellino, M.; Boffo, S.; Di Carlo, A.; Di Domenico, M. The Enigmatic Role of Matrix Metalloproteinases in Epithelial-to-Mesenchymal Transition of Oral Squamous Cell Carcinoma: Implications and Nutraceutical Aspects. J. Cell. Biochem. 2019, 120, 6813–6819. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. A Natural Solution for Obesity: Bioactives for the Prevention and Treatment of Weight Gain. A Review. Nutr. Neurosci. 2015, 18, 49–65. [Google Scholar] [CrossRef]
- Hadváry, P.; Lengsfeld, H.; Wolfer, H. Inhibition of Pancreatic Lipase in Vitro by the Covalent Inhibitor Tetrahydrolipstatin. Biomed. J. 1988, 256, 357–361. [Google Scholar] [CrossRef]
- Douglas, A.; Douglas, J.G.; Robertson, C.E.; Munro, J.F. Plasma Phentermine Levels, Weight Loss and Side-Effects. Int. J. Obes. 1983, 7, 591–595. [Google Scholar]
- Birari, R.B.; Bhutani, K.K. Pancreatic Lipase Inhibitors from Natural Sources: Unexplored Potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef]
- Tenore, G.C.; Carotenuto, A.; Caruso, D.; Buonomo, G.; D’Avino, M.; Brancaccio, D.; Ciampaglia, R.; Maisto, M.; Schisano, C.; Novellino, E. A Nutraceutical Formulation Based on Annurca Apple Polyphenolic Extract Is Effective on Intestinal Cholesterol Absorption: A Randomised, Placebo-Controlled, Crossover Study. PharmaNutrition 2018, 6, 85–94. [Google Scholar] [CrossRef]
- Schiano, E.; Piccolo, V.; Novellino, E.; Maisto, M.; Iannuzzo, F.; Summa, V.; Tenore, G.C. Thinned Nectarines, an Agro-Food Waste with Antidiabetic Potential: HPLC-HESI-MS/MS Phenolic Characterization and In Vitro Evaluation of Their Beneficial Activities. Foods 2022, 11, 1010. [Google Scholar] [CrossRef]
- Schiano, E.; Maisto, M.; Piccolo, V.; Novellino, E.; Annunziata, G.; Ciampaglia, R.; Montesano, C.; Croce, M.; Caruso, G.; Iannuzzo, F.; et al. Beneficial Contribution to Glucose Homeostasis by an Agro-Food Waste Product Rich in Abscisic Acid: Results from a Randomized Controlled Trial. Foods 2022, 11, 2637. [Google Scholar] [CrossRef] [PubMed]
- Schisano, C.; Narciso, V.; Maisto, M.; Annunziata, G.; Grieco, P.; Sommella, E.M.; Tenore, G.C.; Novellino, E. In Vitro Effects of Protein Fractions from Controne Beans (Phaseolus vulgaris L. Ecotype Controne) on Intestinal Permeability, ACE and α-Amylase Activities. Eur. Food Res. Technol. 2019, 245, 2311–2322. [Google Scholar] [CrossRef]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Hassan, S.T.S.; Tenore, G.C.; Novellino, E. Effect of Grape Pomace Polyphenols with or without Pectin on TMAO Serum Levels Assessed by LC/MS-Based Assay: A Preliminary Clinical Study on Overweight/Obese Subjects. Front. Pharmacol. 2019, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Ciampaglia, R.; Maisto, M.; D’avino, M.; Caruso, D.; Tenore, G.C.; Novellino, E. Taurisolo®, a Grape Pomace Polyphenol Nutraceutical Reducing the Levels of Serum Biomarkers Associated with Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 697272. [Google Scholar] [CrossRef]
- Maisto, M.; Schiano, E.; Novellino, E.; Piccolo, V.; Iannuzzo, F.; Salviati, E.; Summa, V.; Annunziata, G.; Tenore, G.C. Application of a Rapid and Simple Technological Process to Increase Levels and Bioccessibility of Free Phenolic Compounds in Annurca Apple Nutraceutical Product. Foods 2022, 11, 1453. [Google Scholar] [CrossRef]
- Maisto, M.; Annunziata, G.; Schiano, E.; Piccolo, V.; Iannuzzo, F.; Santangelo, R.; Ciampaglia, R.; Tenore, G.C.; Novellino, E.; Grieco, P. Potential Functional Snacks: Date Fruit Bars Supplemented by Different Species of Lactobacillus spp. Foods 2021, 10, 1760. [Google Scholar] [CrossRef]
- Riccio, G.; Maisto, M.; Bottone, S.; Badolati, N.; Rossi, G.B.; Tenore, G.C.; Stornaiuolo, M.; Novellino, E. WNT Inhibitory Activity of Malus Pumila Miller Cv Annurca and Malus Domestica Cv Limoncella Apple Extracts on Human Colon-Rectal Cells Carrying Familial Adenomatous Polyposis Mutations. Nutrients 2017, 9, 1262. [Google Scholar] [CrossRef]
- Iannuzzo, F.; Piccolo, V.; Novellino, E.; Schiano, E.; Salviati, E.; Summa, V.; Campiglia, P.; Tenore, G.C.; Maisto, M. A Food-Grade Method for Enhancing the Levels of Low Molecular Weight Proanthocyanidins with Potentially High Intestinal Bioavailability. Int. J. Mol. Sci. 2022, 23, 13557. [Google Scholar] [CrossRef]
- De Cicco, P.; Maisto, M.; Tenore, G.C.; Ianaro, A. Olive Leaf Extract, from Olea europaea L., Reduces Palmitate-Induced Inflammation via Regulation of Murine Macrophages Polarization. Nutrients 2020, 12, 3663. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, W.; Chen, H.; Fang, B.; Qiu, Y.; Chen, X.; Chen, L.; Shu, S.; Zhang, Y.; Zhao, Y.; et al. Design, Synthesis, and Structure-Activity Relationships of 2-Benzylidene-1-Indanone Derivatives as Anti-Inflammatory Agents for Treatment of Acute Lung Injury. Drug Des. Dev. Ther. 2018, 12, 887–899. [Google Scholar] [CrossRef]
- Michalkova, R.; Mirossay, L.; Gazdova, M.; Kello, M.; Mojzis, J. Molecular Mechanisms of Antiproliferative Effects of Natural Chalcones. Cancers 2021, 13, 2730. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Lal, K.; Kumar, L.; Kumar, A.; Kumar, A.; Paul, A.K.; Kumar, R. Synthesis, Crystal Structure and Antimicrobial Potential of Some Fluorinated Chalcone-1,2,3-Triazole Conjugates. Eur. J. Med. Chem. 2018, 155, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The Bioavailability, Extraction, Biosynthesis and Distribution of Natural Dihydrochalcone: Phloridzin. Int. J. Mol. Sci. 2021, 22, 962. [Google Scholar] [CrossRef] [PubMed]
- Maisto, M.; Piccolo, V.; Novellino, E.; Schiano, E.; Iannuzzo, F.; Ciampaglia, R.; Summa, V.; Tenore, G.C. Optimization of Phlorizin Extraction from Annurca Apple Tree Leaves Using Response Surface Methodology. Antioxidants 2022, 11, 1933. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary Chalcones with Chemopreventive and Chemotherapeutic Potential. Genes Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef]
- Israf, D.A.; Khaizurin, T.A.; Syahida, A.; Lajis, N.H.; Khozirah, S. Cardamonin Inhibits COX and INOS Expression via Inhibition of P65NF-ΚB Nuclear Translocation and Iκ-B Phosphorylation in RAW 264.7 Macrophage Cells. Mol. Immunol. 2007, 44, 673–679. [Google Scholar] [CrossRef]
- Yadav, V.R.; Prasad, S.; Sung, B.; Aggarwal, B.B. The Role of Chalcones in Suppression of NF-ΚB-Mediated Inflammation and Cancer. Int. Immunopharmacol. 2011, 11, 295–309. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Daouk, R.; Azar, J.; Sapudom, J.; Teo, J.C.M.; Abou-Kheir, W.; Al-Sayegh, M. Modeling Adipogenesis: Current and Future Perspective. Cells 2020, 9, 2326. [Google Scholar] [CrossRef]
- Zhang, T.; Yamamoto, N.; Yamashita, Y.; Ashida, H. The Chalcones Cardamonin and Flavokawain B Inhibit the Differentiation of Preadipocytes to Adipocytes by Activating ERK. Arch. Biochem. Biophys. 2014, 554, 44–54. [Google Scholar] [CrossRef]
- Gesta, S.; Tseng, Y.H.; Kahn, C.R. Developmental Origin of Fat: Tracking Obesity to Its Source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the Pleiotropic Role of White Adipose Tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef]
- Cinti, S. The Adipose Organ at a Glance. DMM Dis. Models Mech. 2012, 5, 588–594. [Google Scholar] [CrossRef]
- Wu, J.; Cohen, P.; Spiegelman, B.M. Adaptive Thermogenesis in Adipocytes: Is Beige the New Brown? Genes Dev. 2013, 27, 234–250. [Google Scholar] [CrossRef]
- Seo, Y.J.; Jin, H.; Lee, K.; Song, J.H.; Chei, S.; Oh, H.J.; Oh, J.H.; Lee, B.Y. Cardamonin Suppresses Lipogenesis by Activating Protein Kinase A-Mediated Browning of 3T3-L1 Cells. Phytomedicine 2019, 65, 153064. [Google Scholar] [CrossRef]
- Rozmer, Z.; Perjési, P. Naturally Occurring Chalcones and Their Biological Activities. Phytochem. Rev. 2016, 15, 87–120. [Google Scholar] [CrossRef]
- Quan, H.Y.; Baek, N.I.; Chung, S.H. Licochalcone a Prevents Adipocyte Differentiation and Lipogenesis via Suppression of Peroxisome Proliferator-Activated Receptor γ and Sterol Regulatory Element-Binding Protein Pathways. J. Agric. Food Chem. 2012, 60, 5112–5120. [Google Scholar] [CrossRef]
- Lee, H.E.; Yang, G.; Han, S.H.; Lee, J.H.; An, T.J.; Jang, J.K.; Lee, J.Y. Anti-Obesity Potential of Glycyrrhiza Uralensis and Licochalcone A through Induction of Adipocyte Browning. Biochem. Biophys. Res. Commun. 2018, 503, 2117–2123. [Google Scholar] [CrossRef]
- Won, S.R.; Kim, S.K.; Kim, Y.M.; Lee, P.H.; Ryu, J.H.; Kim, J.W.; Rhee, H.I. Licochalcone A: A Lipase Inhibitor from the Roots of Glycyrrhiza Uralensis. Food Res. Int. 2007, 40, 1046–1050. [Google Scholar] [CrossRef]
- Wu, S.C.; Yang, Z.Q.; Liu, F.; Peng, W.J.; Qu, S.Q.; Li, Q.; Song, X.B.; Zhu, K.; Shen, J.Z. Antibacterial Effect and Mode of Action of Flavonoids From Licorice Against Methicillin-Resistant Staphylococcus Aureus. Front. Microbiol. 2019, 10, 2489. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Wu, W.; Zhang, Y.; Pan, X.; Duan, J. Rapid Screening of Lipase Inhibitors in Licorice Extract by Using Porcine Pancreatic Lipase Immobilized on Fe3O4 magnetic Nanoparticles. Food Funct. 2021, 12, 5650–5657. [Google Scholar] [CrossRef] [PubMed]
- Annie-Mathew, A.S.; Prem-Santhosh, S.; Jayasuriya, R.; Ganesh, G.; Ramkumar, K.M.; Sarada, D.V.L.; Abdallah, B.M.; Ali, E.M.; Hemmeryckx, B.; Vranckx, C.; et al. The Pivotal Role of Nrf2 Activators in Adipocyte Biology. Pharmacol. Res. 2021, 25, 209–222. [Google Scholar] [CrossRef]
- Yang, J.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Inhibitory Effects of Butein on Adipogenesis through Upregulation of the Nrf2/HO-1 Pathway in 3T3-L1 Adipocytes. Prev. Nutr. Food Sci. 2017, 22, 306–311. [Google Scholar] [CrossRef]
- Kim, B.R.; Lee, G.Y.; Yu, H.; Maeng, H.J.; Oh, T.J.; Kim, K.M.; Moon, J.H.; Lim, S.; Jang, H.C.; Choi, S.H.; et al. Canonical and Non-Canonical Mechanisms of Nrf2 Activation. Biochem. Biophys. Res. Commun. 2018, 497, 92–99. [Google Scholar] [CrossRef]
- Pi, J.; Leung, L.; Xue, P.; Wang, W.; Hou, Y.; Liu, D.; Yehuda-Shnaidman, E.; Lee, C.; Lau, J.; Kurtz, T.W.; et al. Deficiency in the Nuclear Factor E2-Related Factor-2 Transcription Factor Results in Impaired Adipogenesis and Protects against Diet-Induced Obesity. J. Biol. Chem. 2010, 285, 9292–9300. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Ndisang, J.F. Role of Heme Oxygenase in Inflammation, Insulin-Signalling, Diabetes and Obesity. Mediat. Inflamm. 2010, 2010, 14–20. [Google Scholar] [CrossRef]
- Huang, J.Y.; Chiang, M.T.; Yet, S.F.; Chau, L.Y. Myeloid Heme Oxygenase-1 Haploinsufficiency Reduces High Fat Diet-Induced Insulin Resistance by Affecting Adipose Macrophage Infiltration in Mice. PLoS ONE 2012, 7, e38626. [Google Scholar] [CrossRef]
- Wang, Z.; Ka, S.O.; Lee, Y.; Park, B.H.; Bae, E.J. Butein Induction of HO-1 by P38 MAPK/Nrf2 Pathway in Adipocytes Attenuates High-Fat Diet Induced Adipose Hypertrophy in Mice. Eur. J. Pharmacol. 2017, 799, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Song, N.J.; Yoon, H.J.; Kim, K.H.; Jung, S.R.; Jang, W.S.; Seo, C.R.; Lee, Y.M.; Kweon, D.H.; Hong, J.W.; Lee, J.S.; et al. Butein Is a Novel Anti-Adipogenic Compound. J. Lipid Res. 2013, 54, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A Nutrient and Energy Sensor That Maintains Energy Homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Ceddia, R.B. The Role of AMP-Activated Protein Kinase in Regulating White Adipose Tissue Metabolism. Mol. Cell. Endocrinol. 2013, 366, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Sung, J.; Yang, J.; Jeong, H.S.; Lee, J. Butein Inhibits Adipocyte Differentiation by Modulating the AMPK Pathway in 3T3-L1 Cells. J. Food Biochem. 2018, 42, e12441. [Google Scholar] [CrossRef]
- Ghosh, D.; Parida, P. Multipotential Therapeutic Bioactive Compound: Panduratin A. Everymans Sci. 2020, 55, 24–29. [Google Scholar]
- Kim, D.; Lee, M.S.; Jo, K.; Lee, K.E.; Hwang, J.K. Therapeutic Potential of Panduratin A, LKB1-Dependent AMP-Activated Protein Kinase Stimulator, with Activation of PPARα/δ for the Treatment of Obesity. Diabetes Obes. Metab. 2011, 13, 584–593. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, M.S.; Sa, B.K.; Kim, M.B.; Hwang, J.K. Boesenbergia Pandurata Attenuates Diet-Induced Obesity by Activating AMP-Activated Protein Kinase and Regulating Lipid Metabolism. Int. J. Mol. Sci. 2012, 13, 994–1005. [Google Scholar] [CrossRef]
- Cho, S.; Kim, S.; Jin, Z.; Yang, H.; Han, D.; Baek, N.I.; Jo, J.; Cho, C.W.; Park, J.H.; Shimizu, M.; et al. Isoliquiritigenin, a Chalcone Compound, Is a Positive Allosteric Modulator of GABA A Receptors and Shows Hypnotic Effects. Biochem. Biophys. Res. Commun. 2011, 413, 637–642. [Google Scholar] [CrossRef]
- Chen, X.; Cai, X.; Le, R.; Zhang, M.; Gu, X.; Shen, F.; Hong, G.; Chen, Z. Isoliquiritigenin Protects against Sepsis-Induced Lung and Liver Injury by Reducing Inflammatory Responses. Biochem. Biophys. Res. Commun. 2018, 496, 245–252. [Google Scholar] [CrossRef]
- Peng, F.; Du, Q.; Peng, C.; Wang, N.; Tang, H.; Xie, X.; Shen, J.; Chen, J. A Review: The Pharmacology of Isoliquiritigenin. Phytother. Res. 2015, 29, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liang, J.; Zhang, J.; Wang, Y.; Chai, X. Natural Chalcones in Chinese Materia Medica: Licorice. Evid. Based Complement. Altern. Med. 2020, 2020, 3821248. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Liu, W.; Liang, B.; Shi, L.; Yang, S.; Meng, J.; Chang, J.; Hu, X.; Zhang, R.; Xing, D. Inhibitory Effect of Isoliquiritigenin in Niemann-Pick C1-Like 1-Mediated Cholesterol Uptake. Molecules 2022, 27, 7494. [Google Scholar] [CrossRef]
- White, Z.; Theret, M.; Milad, N.; Tung, L.W.; Chen, W.W.H.; Sirois, M.G.; Rossi, F.; Bernatchez, P. Cholesterol Absorption Blocker Ezetimibe Prevents Muscle Wasting in Severe Dysferlin-Deficient and Mdx Mice. J. Cachexia Sarcopenia Muscle 2022, 13, 544–560. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Choe, Y.G.; Kim, J.H.; Chang, K.T.; Lee, H.S.; Lee, D.S. Isoliquiritigenin Impairs Insulin Signaling and Adipocyte Differentiation through the Inhibition of Protein-Tyrosine Phosphatase 1B Oxidation in 3T3-L1 Preadipocytes. Food Chem. Toxicol. 2016, 93, 5–12. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Bashan, N.; Kovsan, J.; Kachko, I.; Ovadia, H.; Rudich, A. Positive and Negative Regulation of Insulin Signaling by Reactive Oxygen and Nitrogen Species. Physiol. Rev. 2009, 89, 27–71. [Google Scholar] [CrossRef]
- Moon, H.; Choi, J.W.; Song, B.W.; Kim, I.K.; Lim, S.; Lee, S.; Hwang, K.C.; Kim, S.W. Isoliquiritigenin Enhances the Beige Adipocyte Potential of Adipose-Derived Stem Cells by Jnk Inhibition. Molecules 2020, 25, 5660. [Google Scholar] [CrossRef]
- Loh, R.K.C.; Kingwell, B.A.; Carey, A.L. Human Brown Adipose Tissue as a Target for Obesity Management; beyond Cold-Induced Thermogenesis. Obes. Rev. 2017, 18, 1227–1242. [Google Scholar] [CrossRef]
- Bolasco, A.; Carradori, S.; Fioravanti, R. Focusing on New Monoamine Oxidase Inhibitors. Expert Opin. Ther. Pat. 2010, 20, 909–939. [Google Scholar] [CrossRef]
- Prajapati, R.; Seong, S.H.; Park, S.E.; Paudel, P.; Jung, H.A.; Choi, J.S. Isoliquiritigenin, a Potent Human Monoamine Oxidase Inhibitor, Modulates Dopamine D1, D3, and Vasopressin V1A Receptors. Sci. Rep. 2021, 11, 23528. [Google Scholar] [CrossRef] [PubMed]
- Samuels, J.S.; Shashidharamurthy, R.; Rayalam, S. Novel Anti-Obesity Effects of Beer Hops Compound Xanthohumol: Role of AMPK Signaling Pathway. Nutr. Metab. 2018, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Inoue, J.; Shimizu, M.; Sato, R. Xanthohumol Improves Diet-Induced Obesity and Fatty Liver by Suppressing Sterol Regulatory Element-Binding Protein (SREBP) Activation. J. Biol. Chem. 2015, 290, 20565–20579. [Google Scholar] [CrossRef]
- Rayalam, S.; Yang, J.-Y.; Della-Fera, M.A.; Park, H.J.; Ambati, S.; Baile, C.A. Anti-Obesity Effects of Xanthohumol Plus Guggulsterone in 3T3-L1 Adipocytes. J. Med. Food 2009, 12, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.E.; Whyand, T.; Caplin, M.E. Benefits of Xanthohumol in Hyperlipidaemia, Obesity and Type 2 Diabetes Mellitus: A Review. J. Obes. Chronic Dis. 2019, 3, 14–18. [Google Scholar] [CrossRef]
- Hanif, N.; Iswantini, D.; Hioki, Y.; Murni, A.; Kita, M.; Tanaka, J. Flavokawains, Plant-Derived Chalcones, Inhibit Differentiation of Murine Pre-Adipocytes. Chem. Lett. 2022, 51, 54–57. [Google Scholar] [CrossRef]
- Zhang, T.; Sawada, K.; Yamamoto, N.; Ashida, H. 4-Hydroxyderricin and Xanthoangelol from Ashitaba (Angelica Keiskei) Suppress Differentiation of Preadiopocytes to Adipocytes via AMPK and MAPK Pathways. Mol. Nutr. Food Res. 2013, 57, 1729–1740. [Google Scholar] [CrossRef]
- Kim, J.H.; Son, Y.K.; Kim, G.H.; Hwang, K.H. Xanthoangelol and 4-Hydroxyderricin Are the Major Active Principles of the Inhibitory Activities against Monoamine Oxidases on Angelica Keiskei K. Biomol. Ther. 2013, 21, 234–240. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, M.; Song, H.; Lee, C.S.; Oh, W.K.; Mook-Jung, I.; Chung, S.S.; Park, K.S. DMC (2′,4′-Dihydroxy-6′-Methoxy-3′,5′-Dimethylchalcone) Improves Glucose Tolerance as a Potent AMPK Activator. Metabolism 2016, 65, 533–542. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Chang, F.R.; Tsai, Y.H.; Wu, Y.C.; Hsieh, T.J. 2-Bromo-4′-Methoxychalcone and 2-Iodo-4′-Methoxychalcone Prevent Progression of Hyperglycemia and Obesity Via 5′-Adenosine-Monophosphate-Activated Protein Kinase in Diet-Induced Obese Mice. Int. J. Mol. Sci. 2018, 19, 2763. [Google Scholar] [CrossRef]
- Song, N.J.; Choi, S.; Rajbhandari, P.; Chang, S.H.; Kim, S.; Vergnes, L.; Kwon, S.M.; Yoon, J.H.; Lee, S.; Ku, J.M.; et al. Prdm4 Induction by the Small Molecule Butein Promotes White Adipose Tissue Browning. Nat. Chem. Biol. 2016, 12, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Hemmeryckx, B.; Vranckx, C.; Bauters, D.; Lijnen, H.R.; Scroyen, I. Does Butein Affect Adipogenesis? Adipocyte 2019, 8, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Farias-Pereira, R.; Zhang, Z.; Park, C.S.; Kim, D.; Kim, K.H.; Park, Y. Butein Inhibits Lipogenesis in Caenorhabditis Elegans. BioFactors 2020, 46, 777–787. [Google Scholar] [CrossRef]
- Quan, H.Y.; Kim, S.J.; Kim, D.Y.; Jo, H.K.; Kim, G.W.; Chung, S.H. Licochalcone A Regulates Hepatic Lipid Metabolism through Activation of AMP-Activated Protein Kinase. Fitoterapia 2013, 86, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.-J.; Lee, Y.-K.; Ting, N.-C.; Chen, Y.-L.; Shen, S.-C.; Wu, S.-J.; Huang, W.-C. Protective Effects of Licochalcone A Ameliorates Obesity and Non-Alcoholic Fatty Liver Disease Via Promotion of the Sirt-1/AMPK Pathway in Mice Fed a High-Fat Diet. Cell 2019, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Zhang, C.L.; Yu, R.T.; Cho, H.K.; Nelson, M.C.; Bayuga-Ocampo, C.R.; Ham, J.; Kang, H.; Evans, R.M. Regulation of Muscle Fiber Type and Running Endurance by PPARδ. PLoS Biol. 2004, 2, e294. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kwon, E.Y.; Choi, M.S. Dietary Isoliquiritigenin at a Low Dose Ameliorates Insulin Resistance and NAFLD in Diet-Induced Obesity in C57BL/6J Mice. Int. J. Mol. Sci. 2018, 19, 3281. [Google Scholar] [CrossRef]
- Ishibashi, R.; Furusawa, Y.; Honda, H.; Watanabe, Y.; Fujisaka, S.; Nishikawa, M.; Ikushiro, S.; Kurihara, S.; Tabuchi, Y.; Tobe, K.; et al. Isoliquiritigenin Attenuates Adipose Tissue Inflammation and Metabolic Syndrome by Modifying Gut Bacteria Composition in Mice. Mol. Nutr. Food Res. 2022, 66, e2101119. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Wu, T.R.; Lin, C.S.; Chang, C.J.; Lin, T.L.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Lu, C.C.; Young, J.D.; Lai, H.C. Gut Commensal Parabacteroides Goldsteinii Plays a Predominant Role in the Anti-Obesity Effects of Polysaccharides Isolated from Hirsutella Sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Fahimi, P.; Matta, C.F. On the Power per Mitochondrion and the Number of Associated Active ATP synthases. Phys. Biol. 2021, 18, 04LT01. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, A.P.A.; da Silva, A.S.R.; Muñoz, V.R.; Ropelle, E.R.; Pauli, J.R. Mitochondrial Dysfunction Plays an Essential Role in Remodeling Aging Adipose Tissue. Mech. Ageing Dev. 2021, 200, 111598. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, J.S.; Legette, L.C.L.; Miranda, C.L.; Jiang, Y.; Stevens, J.F. A Metabolomics-Driven Elucidation of the Anti-Obesity Mechanisms of Xanthohumol. J. Biol. Chem. 2013, 288, 19000–19013. [Google Scholar] [CrossRef]
- Legette, L.L.; Moreno Luna, A.Y.; Reed, R.L.; Miranda, C.L.; Bobe, G.; Proteau, R.R.; Stevens, J.F. Xanthohumol Lowers Body Weight and Fasting Plasma Glucose in Obese Male Zucker Fa/Fa Rats. Phytochemistry 2013, 91, 236–241. [Google Scholar] [CrossRef]
- Khayyal, M.T.; El-Hazek, R.M.; El-Sabbagh, W.A.; Frank, J.; Behnam, D.; Abdel-Tawab, M. Micellar Solubilization Enhances the Anti-Inflammatory Effect of Xanthohumol. Phytomedicine 2020, 71, 4–9. [Google Scholar] [CrossRef]
- Mahli, A.; Seitz, T.; Freese, K.; Frank, J.; Weiskirchen, R.; Abdel-Tawab, M.; Behnam, D.; Hellerbrand, C. Therapeutic Application of Micellar Solubilized Xanthohumol in a Western-Type Diet-Induced Mouse Model of Obesity, Diabetes and Non-Alcoholic Fatty Liver Disease. Cells 2019, 8, 359. [Google Scholar] [CrossRef]

| Chalcone | Cell Model | Dose | Mechanism | Ref. |
|---|---|---|---|---|
| Cardamomin | 3T3-L1 | 10 or 30 µM | PPARγ ↓, FABP4 ↓, C/EBPα ↓, PRDM16 ↑, PGC1α ↑, UCP1 ↑, ERK ↑, (PKA)-mediated browning ↑ | [31] |
| 3T3-L1 | 3, 6, 12, 25, and 50 µM | C/EBPα ↓, FABP4 ↓, LPAATθ ↓, DGAT1 ↓, SREBP1 ↓, FAS ↓ | [38] | |
| Licochalcone A | 3T3-L1 | 5 and 10 µM | PPARγ ↓, C/EBPα ↓, SREBP1c ↓, FAS ↓, SCD1 ↓, GPAT ↓ CPT1 ↑, ACC ↓, AMPK ↑, PPAR-α ↑, UCP1 ↑, | [40] |
| - | 35 mg/mL | inhibition of the pancreatic lipase enzyme | [42] | |
| Butein | 3T3-L1 | 5, 10, and 25 μM | PPARγ ↓, C/EBPα ↓, Nrf2 ↑, HO-1 ↑ | [46] |
| 3T3-L1 | 30 µM | HO-1 ↑ | [52] | |
| C3H10T1/2 cells | 10 mM | Lipid accumulation ↓, PPARγ ↓, aP2 ↓, and LPL 100% | [53] | |
| PPARγ, aP2, and LPL 50% ↓ | ||||
| 3T3-L1 | 1–40 µM | AMPK ↑, PPARγ ↓, C/EBPα ↓, GPAT-1 ↓, CPT1 ↑, ACC ↑ | [56] | |
| Panduratin A | 3T3-L1, HepG2, and L6 skeletal muscle cells | - | Triglyceride accumulation ↓, AMPK ↑, PPARγ ↓, C/EBPα ↓, ACC ↓, FAS ↓, SREBP1c ↓, PPARα ↑, PGC-1α ↑, CPT-1L ↑, CPT-1M ↑, UCPs ↑ | [59] |
| Isoliquiritin | Caco-2, HepG2 | 100 μmol/L | Cholesterol lowering, NPC1L1 ↓, HDL catabolism ↑ | [64] |
| 3T3-L1 | 100 µM | Insulin-stimulated ROS production and adipocyte differentiation ↓, superoxide generation ↓, FABP4 ↓, GLUT4 ↓, PPARγ ↓, C/EBPα ↓, PTP1B oxidation ↓, AKT phosphorylation ↓ | [66] | |
| Human adipose-derived stem cells (hASCs) | UCP1 ↑, PRDM16 ↑, JNK ↑ | [69] | ||
| Xanthohumol | 3T3-L1 and primary human subcutaneous preadipocytes | CIDE-A ↑, TBX-1 ↑, UCP1 ↑, ACC ↓, HSL ↑, PGC-1α ↑ | [74] | |
| Huh-7 | ||||
| 3T3-L1 | 1.5 mM | [75] | ||
| Flavokawains | 3T3-L1 | 10 µg/mL | Adipocyte differentiation ↓, phosphorylation of ERK ↑, lipid accumulation ↓, (C/EBP)-β ↓, C/EBPα ↓, PPARγ ↓ | [77] |
| Xanthoangelol | 3T3-L1 | 1, 5, 10, and 30 µM | C/EBP ↓, C/EBP ↓, PPARγ ↓ | [78] |
| 4-Hydroxyderricin | 3T3-L1 | 1, 5, 10, and 30 µM | Glycerol-3-phosphate acyl transferase-1 ↓, CPT ↑ | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maisto, M.; Marzocchi, A.; Keivani, N.; Piccolo, V.; Summa, V.; Tenore, G.C. Natural Chalcones for the Management of Obesity Disease. Int. J. Mol. Sci. 2023, 24, 15929. https://doi.org/10.3390/ijms242115929
Maisto M, Marzocchi A, Keivani N, Piccolo V, Summa V, Tenore GC. Natural Chalcones for the Management of Obesity Disease. International Journal of Molecular Sciences. 2023; 24(21):15929. https://doi.org/10.3390/ijms242115929
Chicago/Turabian StyleMaisto, Maria, Adua Marzocchi, Niloufar Keivani, Vincenzo Piccolo, Vincenzo Summa, and Gian Carlo Tenore. 2023. "Natural Chalcones for the Management of Obesity Disease" International Journal of Molecular Sciences 24, no. 21: 15929. https://doi.org/10.3390/ijms242115929