Lipid Nanoparticles: An Effective Tool to Improve the Bioavailability of Nutraceuticals
Abstract
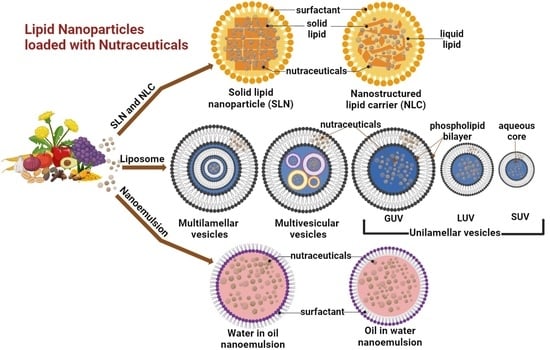
:1. Introduction
1.1. Methods of Preparation for SLN and NLC
1.1.1. High-Pressure Homogenization (HPH)
1.1.2. High Shear Homogenization and Ultrasonication
1.1.3. Microemulsion Technique
1.1.4. Phase Inversion Temperature (PIT) Technique
1.1.5. Double Emulsion Technique
1.1.6. Solvent Injection Technique
1.1.7. Solvent Evaporation Method
1.1.8. Precipitation Method
1.1.9. Coacervation Method
1.2. Methods of Preparation for Liposomes
1.2.1. Thin-Film Method
1.2.2. Proliposome Method
1.2.3. Injection Methods
1.2.4. Emulsification Method
1.3. Methods of Preparation of Nanoemulsion
1.3.1. High-Energy Methods
High-Pressure Homogenization
Microfluidization
Ultrasonication
1.3.2. Low-Energy Methods
1.3.3. Self-Nanoemulsification Method
1.4. Characterization of LNPs
2. Phytochemicals
2.1. Lycopene
2.2. β-Carotenoid
2.3. Eugenol
2.4. Curcumin
2.5. Resveratrol (Rvt)
2.6. Hesperetin
2.7. Capsaicin
2.8. Naringenin (Nar)
2.9. Baicalin
2.10. β-Sitosterol
2.11. Sesamol
3. Functional Foods
3.1. Vitamin B12
3.2. Vitamin A
3.3. Vitamin C
3.4. Vitamin D
3.5. Vitamin E
3.6. Vitamin K
3.7. Omega(ω)-3 Polyunsaturated Fatty Acids
4. Dietary Supplements
4.1. Melatonin
4.2. Coenzyme Q10
5. Herbs and Spices
6. Current Gaps, Challenges, and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar]
- Rishi, R. Nutraceuticals: Borderline between food and drug. Pharma Rev. 2006, 2, 51–53. [Google Scholar]
- Gulati, O.P.; Ottaway, P.B. Legislation relating to nutraceuticals in the European Union with a particular focus on botanical-sourced products. Toxicology 2006, 221, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Verma, R.K.; Saraf, S.A. Nutraceuticals: New era of medicine and health. Asian J. Pharm. Clin. Res. 2010, 3, 11–15. [Google Scholar]
- Rahman, A.; Uahengo, V.; Likius, D. Mini review on emerging methods of preparation of liposome and its application as Liposome drug delivery systems. Open J. Pharmacol. Pharmacother. 2018, 3, 5–21. [Google Scholar]
- Huang, Q.; Given, P. Micro/Nano Encapsulation of Active Food Ingredients; American Chemical Society: Washington, DC, USA, 2009. [Google Scholar]
- Huang, Q.; Yu, H.; Ru, Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J. Food Sci. 2010, 75, R50–R57. [Google Scholar] [CrossRef]
- Üner, M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems. Die Pharm.-Int. J. Pharm. Sci. 2006, 61, 375–386. [Google Scholar]
- Cirri, M.; Bragagni, M.; Mennini, N.; Mura, P. Development of a new delivery system consisting in “drug–in cyclodextrin–in nanostructured lipid carriers” for ketoprofen topical delivery. Eur. J. Pharm. Biopharm. 2012, 80, 46–53. [Google Scholar] [CrossRef]
- Schäfer-Korting, M.; Mehnert, W.; Korting, H.-C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef]
- Esposito, E.; Mariani, P.; Ravani, L.; Contado, C.; Volta, M.; Bido, S.; Drechsler, M.; Mazzoni, S.; Menegatti, E.; Morari, M. Nanoparticulate lipid dispersions for bromocriptine delivery: Characterization and in vivo study. Eur. J. Pharm. Biopharm. 2012, 80, 306–314. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Glatter, O. Characterization and potential applications of nanostructured aqueous dispersions. Adv. Colloid Interface Sci. 2009, 147, 333–342. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional polymeric nanoplatforms for brain diseases diagnosis, therapy and theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, I.F.; Hussein, M.Z. Synthesis and technology of nanoemulsion-based pesticide formulation. Nanomaterials 2020, 10, 1608. [Google Scholar] [CrossRef]
- Tarhan, O.; Spotti, M.J. Nutraceutical delivery through nano-emulsions: General aspects, recent applications and patented inventions. Colloids Surf. B Biointerfaces 2021, 200, 111526. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Research, P. Nutraceuticals Market Size, Growth, Trends, Report by 2030; Precedence Research: Ottawa, ON, Canada, 2021. [Google Scholar]
- Research, G.V. Nanomedicine Market Size, Share & Trends Analysis Report by Application (Drug Delivery), by Indication (Clinical Oncology, Infectious Diseases), by Molecule Type, by Region, and Segment Forecasts, 2023–2030; 978-1-68038-942-5; Grand View Research: San Francisco, CA, USA, 2023; p. 150. [Google Scholar]
- Insights, G.M. Nutraceutical Market-By Product (Functional Foods {Baby Food, Cereals}, Dietary Supplements), Form (Capsules, Tablets, Gummies), Condition (Weight Management, Joint Health, Skin, Hair), Distribution Channel (Pharmacies), Global Forecast, 2023–2032; GMI5297; Global Market Insights: Selbyville, DE, USA, 2023; p. 270. [Google Scholar]
- Lippacher, A.; Müller, R.; Mäder, K. Investigation on the viscoelastic properties of lipid based colloidal drug carriers. Int. J. Pharm. 2000, 196, 227–230. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery–A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Basha, S.K.; Dhandayuthabani, R.; Muzammil, M.S.; Kumari, V.S. Solid lipid nanoparticles for oral drug delivery. Mater. Today Proc. 2021, 36, 313–324. [Google Scholar] [CrossRef]
- Svilenov, H.; Tzachev, C. Solid lipid nanoparticles–apromising drug delivery system. Nanomedicine 2014, 8, 188–237. [Google Scholar]
- Ren, G.; Sun, Z.; Wang, Z.; Zheng, X.; Xu, Z.; Sun, D. Nanoemulsion formation by the phase inversion temperature method using polyoxypropylene surfactants. J. Colloid Interface Sci. 2019, 540, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Weerapol, Y.; Manmuan, S.; Chaothanaphat, N.; Limmatvapirat, S.; Sirirak, J.; Tamdee, P.; Tubtimsri, S. New Approach for Preparing Solid Lipid Nanoparticles with Volatile Oil-Loaded Quercetin Using the Phase-Inversion Temperature Method. Pharmaceutics 2022, 14, 1984. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid nanoparticles for drug delivery. Adv. NanoBiomed Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Gomaa, E.; Fathi, H.A.; Eissa, N.G.; Elsabahy, M. Methods for preparation of nanostructured lipid carriers. Methods 2022, 199, 3–8. [Google Scholar] [CrossRef]
- Chen, D.-B.; Yang, T.-Z.; Lu, W.-L.; Zhang, Q. In vitro and in vivo study of two types of long-circulating solid lipid nanoparticles containing paclitaxel. Chem. Pharm. Bull. 2001, 49, 1444–1447. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Matinise, N.; Mayedwa, N.; Mongwaketsi, N.; Letsholathebe, D.; Mola, G.; AbdullahAl-Dhabi, N.; Valan Arasu, M.; Henini, M.; Kennedy, J. Evaluation on La2O3 garlanded ceria heterostructured binary metal oxide nanoplates for UV/visible light induced removal of organic dye from urban wastewater. S. Afr. J. Chem. Eng. 2018, 26, 49–60. [Google Scholar]
- Jovanović, A.A.; Balanč, B.D.; Ota, A.; Ahlin Grabnar, P.; Djordjević, V.B.; Šavikin, K.P.; Bugarski, B.M.; Nedović, V.A.; Poklar Ulrih, N. Comparative effects of cholesterol and β-sitosterol on the liposome membrane characteristics. Eur. J. Lipid Sci. Technol. 2018, 120, 1800039. [Google Scholar] [CrossRef]
- Istenič, K.; Cerc Korošec, R.; Poklar Ulrih, N. Encapsulation of (−)-epigallocatechin gallate into liposomes and into alginate or chitosan microparticles reinforced with liposomes. J. Sci. Food Agric. 2016, 96, 4623–4632. [Google Scholar] [CrossRef]
- Gouda, A.; Sakr, O.S.; Nasr, M.; Sammour, O. Ethanol injection technique for liposomes formulation: An insight into development, influencing factors, challenges and applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102174. [Google Scholar] [CrossRef]
- Laloy, E.; Vuillemard, J.-C.; Ackermann, H.-W.; Robin, O. Preparation of liposomes by a simple emulsification technique. Biotechnol. Tech. 1994, 8, 717–722. [Google Scholar] [CrossRef]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for formulation of nanoemulsion drug delivery system: A review. Prev. Nutr. Food Sci. 2019, 24, 225. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Hernández-Ortega, C.; Welti-Chanes, J.; Putnik, P.; Barba, F.J.; Mallikarjunan, K.; Escobedo-Avellaneda, Z.; Roohinejad, S. High pressure processing of food-grade emulsion systems: Antimicrobial activity, and effect on the physicochemical properties. Food Hydrocoll. 2019, 87, 307–320. [Google Scholar] [CrossRef]
- Zaaboul, F.; Raza, H.; Cao, C.; Yuanfa, L. The impact of roasting, high pressure homogenization and sterilization on peanut milk and its oil bodies. Food Chem. 2019, 280, 270–277. [Google Scholar] [CrossRef]
- García-Márquez, E.; Higuera-Ciapara, I.; Espinosa-Andrews, H. Design of fish oil-in-water nanoemulsion by microfluidization. Innov. Food Sci. Emerg. Technol. 2017, 40, 87–91. [Google Scholar] [CrossRef]
- Akbas, E.; Soyler, B.; Oztop, M.H. Formation of capsaicin loaded nanoemulsions with high pressure homogenization and ultrasonication. LWT 2018, 96, 266–273. [Google Scholar] [CrossRef]
- Sneha, K.; Kumar, A. Nanoemulsions: Techniques for the preparation and the recent advances in their food applications. Innov. Food Sci. Emerg. Technol. 2022, 76, 102914. [Google Scholar]
- Chuesiang, P.; Siripatrawan, U.; Sanguandeekul, R.; McLandsborough, L.; McClements, D.J. Optimization of cinnamon oil nanoemulsions using phase inversion temperature method: Impact of oil phase composition and surfactant concentration. J. Colloid Interface Sci. 2018, 514, 208–216. [Google Scholar] [CrossRef]
- Rehman, M.; Khan, M.Z.; Tayyab, M.; Madni, A.; Khalid, Q. Self-Nanoemulsification of Healthy Oils to Enhance the Solubility of Lipophilic Drugs. JoVE 2022, 185, e63995. [Google Scholar] [CrossRef]
- Thaller, A.; Schmauder, L.; Frieß, W.; Winter, G.; Menzen, T.; Hawe, A.; Richter, K. SV-AUC as a stability-indicating method for the characterization of mRNA-LNPs. Eur. J. Pharm. Biopharm. 2023, 182, 152–156. [Google Scholar] [CrossRef]
- Guan, R.; Van Le, Q.; Yang, H.; Zhang, D.; Gu, H.; Yang, Y.; Sonne, C.; Lam, S.S.; Zhong, J.; Jianguang, Z. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere 2021, 271, 129499. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.M.; Patel, K.K.; Dehari, D.; Pandey, N.; Tilak, R.; Agrawal, A.K.; Singh, S. Anacardic acid encapsulated solid lipid nanoparticles for Staphylococcus aureus biofilm therapy: Chitosan and DNase coating improves antimicrobial activity. Drug Deliv. Transl. Res. 2021, 11, 305–317. [Google Scholar] [CrossRef]
- Li, P.; Bukhari, S.N.A.; Khan, T.; Chitti, R.; Bevoor, D.B.; Hiremath, A.R.; SreeHarsha, N.; Singh, Y.; Gubbiyappa, K.S. Apigenin-Loaded Solid Lipid Nanoparticle Attenuates Diabetic Nephropathy Induced by Streptozotocin Nicotinamide Through Nrf2/HO-1/NF-kB Signalling Pathway. Int. J. Nanomed. 2020, 15, 9115–9124. [Google Scholar] [CrossRef]
- Long, Y.; Xiang, Y.; Liu, S.; Zhang, Y.; Wan, J.; Yang, Q.; Cui, M.; Ci, Z.; Li, N.; Peng, W. Baicalin Liposome Alleviates Lipopolysaccharide-Induced Acute Lung Injury in Mice via Inhibiting TLR4/JNK/ERK/NF-κB Pathway. Mediat. Inflamm. 2020, 2020, 8414062. [Google Scholar] [CrossRef]
- Attri, S.; Kumar, A.; Kaur, K.; Kaur, P.; Punj, S.; Bedi, N.; Tuli, H.S.; Arora, S. Assessment of anti-psoriatic activity of bakuchiol-loaded solid lipid nanoparticles-based gel: Design, characterization, and mechanistic insight via NF-kB signaling pathway. Naunyn-Schmiedeberg's Arch. Pharmacol. 2023, 396, 2105–2125. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Y.; Wang, K.; Zhu, X.; Tharkar, P.; Shu, W.; Zhang, T.; Zeng, S.; Zhu, L.; Murray, M.; et al. Compritol solid lipid nanoparticle formulations enhance the protective effect of betulinic acid derivatives in human Müller cells against oxidative injury. Exp. Eye Res. 2022, 215, 108906. [Google Scholar] [CrossRef]
- Andrade, S.; Pereira, M.C.; Loureiro, J.A. Caffeic acid loaded into engineered lipid nanoparticles for Alzheimer's disease therapy. Colloids Surf. B Biointerfaces 2023, 225, 113270. [Google Scholar] [CrossRef]
- Freitas, M.M.; Cavalcante, P.M.; Duarte-Filho, L.A.M.S.; Macedo, C.A.F.; Brito, M.C.; Menezes, P.M.N.; Ribeiro, T.F.; Costa, S.M.; Carvalho, B.A.G.; Ribeiro, F.P.R.A.; et al. Investigation of the relaxing effect of a camphor nanoemulsion on rat isolated trachea. Chem.-Biol. Interact. 2021, 348, 109656. [Google Scholar] [CrossRef]
- Yang, R.; Miao, J.; Shen, Y.; Cai, N.; Wan, C.; Zou, L.; Chen, C.; Chen, J. Antifungal effect of cinnamaldehyde, eugenol and carvacrol nanoemulsion against Penicillium digitatum and application in postharvest preservation of citrus fruit. LWT 2021, 141, 110924. [Google Scholar] [CrossRef]
- Farhadi, A.; Homayouni Tabrizi, M.; Sadeghi, S.; Vala, D.; Khosravi, T. Targeted delivery and anticancer effects of Chrysin-loaded chitosan-folic acid coated solid lipid nanoparticles in pancreatic malignant cells. J. Biomater. Sci. Polym. Ed. 2023, 34, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Sang, N.; Jiang, L.; Wang, Z.; Zhu, Y.; Lin, G.; Li, R.; Zhang, J. Bacteria-targeting liposomes for enhanced delivery of cinnamaldehyde and infection management. Int. J. Pharm. 2022, 612, 121356. [Google Scholar] [CrossRef]
- Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Abu, N.; Mohamad, N.E.; How, C.W.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. In vitro cytotoxicity and anticancer effects of citral nanostructured lipid carrier on MDA MBA-231 human breast cancer cells. Sci. Rep. 2019, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, M.H.; Foudah, A.I.; Alam, A.; Salkini, M.A.; Muharram, M.M.; Labrou, N.E.; Rawat, P. Coumarin-Encapsulated Solid Lipid Nanoparticles as an Effective Therapy against Methicillin-Resistant Staphylococcus aureus. Bioengineering 2022, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Jiang, Z.; Wu, G.; Cai, Z.; Du, Q.; Tao, L.; Zhang, Y.; Chen, Y.; Shen, X. Improving protection effects of eucalyptol via carboxymethyl chitosan-coated lipid nanoparticles on hyperglycaemia-induced vascular endothelial injury in rats. J. Drug Targeting 2021, 29, 520–530. [Google Scholar] [CrossRef]
- Fu, X.; Gao, Y.; Yan, W.; Zhang, Z.; Sarker, S.; Yin, Y.; Liu, Q.; Feng, J.; Chen, J. Preparation of eugenol nanoemulsions for antibacterial activities. RSC Adv. 2022, 12, 3180–3190. [Google Scholar] [CrossRef]
- Senthil Kumar, C.; Thangam, R.; Mary, S.A.; Kannan, P.R.; Arun, G.; Madhan, B. Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells. Carbohydr. Polym. 2020, 231, 115682. [Google Scholar] [CrossRef]
- Subroto, E.; Andoyo, R.; Indiarto, R.; Lembong, E.; Rahmani, F. Physicochemical properties, sensory acceptability, and antioxidant activity of chocolate bar fortified by solid lipid nanoparticles of gallic acid. Int. J. Food Prop. 2022, 25, 1907–1919. [Google Scholar] [CrossRef]
- Simão, D.O.; Honorato, T.D.; Gobo, G.G.; Piva, H.L.; Goto, P.L.; Rolim, L.A.; Turrin, C.-O.; Blanzat, M.; Tedesco, A.C.; Siqueira-Moura, M.P. Preparation and cytotoxicity of lipid nanocarriers containing a hydrophobic flavanone. Colloids Surf. Physicochem. Eng. Asp. 2020, 601, 124982. [Google Scholar] [CrossRef]
- Gostyńska, A.; Czerniel, J.; Kuźmińska, J.; Brzozowski, J.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V.; Stawny, M. Honokiol-Loaded Nanoemulsion for Glioblastoma Treatment: Statistical Optimization, Physicochemical Characterization, and an In Vitro Toxicity Assay. Pharmaceutics 2023, 15, 448. [Google Scholar] [CrossRef]
- Ma, H.L.; Varanda, L.C.; Perussi, J.R.; Carrilho, E. Hypericin-loaded oil-in-water nanoemulsion synthesized by ultrasonication process enhances photodynamic therapy efficiency. J. Photochem. Photobiol. B Biol. 2021, 223, 112303. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Qin, G.; Chang, S.; Jing, Z.; Zhang, Y.; Wang, Y. Antitumor Effect of Hyperoside Loaded in Charge Reversed and Mitochondria-Targeted Liposomes. Int. J. Nanomed. 2021, 16, 3073–3089. [Google Scholar] [CrossRef] [PubMed]
- Moghadaszadeh, M.; Khayyati, M.; Spotin, A.; Norouzi, R.; Pagheh, A.S.; Oliveira, S.M.R.; Pereira, M.d.L.; Ahmadpour, E. Scolicidal and Apoptotic Activities of 5-hydroxy-1, 4-naphthoquinone as a Potent Agent against Echinococcus granulosus Protoscoleces. Pharmaceuticals 2021, 14, 623. [Google Scholar] [CrossRef] [PubMed]
- Nicoleti, L.R.; Di Filippo, L.D.; Duarte, J.L.; Luiz, M.T.; Sábio, R.M.; Chorilli, M. Development, characterization and in vitro cytotoxicity of kaempferol-loaded nanostructured lipid carriers in glioblastoma multiforme cells. Colloids Surf. B Biointerfaces 2023, 226, 113309. [Google Scholar] [CrossRef] [PubMed]
- Mendes Miranda, S.E.; Alcântara Lemos, J.d.; Fernandes, R.S.; Silva, J.d.O.; Ottoni, F.M.; Townsend, D.M.; Rubello, D.; Alves, R.J.; Cassali, G.D.; Ferreira, L.A.M.; et al. Enhanced antitumor efficacy of lapachol-loaded nanoemulsion in breast cancer tumor model. Biomed. Pharmacother. 2021, 133, 110936. [Google Scholar] [CrossRef]
- Silva, L.M.; Marconato, D.G.; Silva, M.P.N.d.; Raposo, N.R.B.; Facchini, G.d.F.S.; Macedo, G.C.; de Sá Teixeira, F.; Salvadori, M.C.B.d.S.; Pinto, P.d.F.; Moraes, J.d.; et al. Licochalcone A-loaded solid lipid nanoparticles improve antischistosomal activity in vitro and in vivo. Nanomedicine 2021, 16, 1641–1655. [Google Scholar] [CrossRef]
- Brenelli, L.B.; Mariutti, L.R.B.; Villares Portugal, R.; de Farias, M.A.; Bragagnolo, N.; Mercadante, A.Z.; Franco, T.T.; Rabelo, S.C.; Squina, F.M. Modified lignin from sugarcane bagasse as an emulsifier in oil-in-water nanoemulsions. Ind. Crops Prod. 2021, 167, 113532. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.; Zhao, S.; Zhang, F.; Meng, X.; Liu, B. Highly stable nanostructured lipid carriers containing candelilla wax for d-limonene encapsulation: Preparation, characterization and antifungal activity. Food Hydrocoll. 2023, 145, 109101. [Google Scholar] [CrossRef]
- Alidadi, H.; Ashtari, A.; Samimi, A.; Karami, M.A.; Khorsandi, L. Myricetin loaded in solid lipid nanoparticles induces apoptosis in the HT-29 colorectal cancer cells via mitochondrial dysfunction. Mol. Biol. Rep. 2022, 49, 8537–8545. [Google Scholar] [CrossRef]
- Wei, X.; Yang, D.; Xing, Z.; Cai, J.; Wang, L.; Zhao, C.; Wei, X.; Jiang, M.; Sun, H.; Zhou, L.; et al. Hepatocyte-targeted delivery using oleanolic acid-loaded liposomes for enhanced hepatocellular carcinoma therapy. Biomater. Sci. 2023, 11, 3952–3964. [Google Scholar] [CrossRef]
- Wang, D.; Yang, F.; Shang, W.; Zhao, Z.; Shen, J.; Cai, H. Paeoniflorin-loaded pH-sensitive liposomes alleviate synovial inflammation by altering macrophage polarity via STAT signaling. Int. Immunopharmacol. 2021, 101, 108310. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wei, S.; Li, Z.; Li, L.; Zhang, X.; Li, C.; Gao, D. Nano magnetic liposomes-encapsulated parthenolide and glucose oxidase for ultra-efficient synergistic antitumor therapy. Nanotechnology 2020, 31, 355104. [Google Scholar] [CrossRef] [PubMed]
- Peczek, S.H.; Tartari, A.P.S.; Zittlau, I.C.; Diedrich, C.; Machado, C.S.; Mainardes, R.M. Enhancing Oral Bioavailability and Brain Biodistribution of Perillyl Alcohol Using Nanostructured Lipid Carriers. Pharmaceuticals 2023, 16, 1055. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, D.; Lin, H.; Jiang, S.; Han, L.; Hou, S.; Lin, S.; Cheng, Z.; Bian, W.; Zhang, X.; et al. Enhanced oral bioavailability and bioefficacy of phloretin using mixed polymeric modified self-nanoemulsions. Food Sci. Nutr. 2020, 8, 3545–3558. [Google Scholar] [CrossRef]
- Hammoud, Z.; Kayouka, M.; Trifan, A.; Sieniawska, E.; Jemâa, J.M.B.; Elaissari, A.; Greige-Gerges, H. Encapsulation of α-Pinene in Delivery Systems Based on Liposomes and Cyclodextrins. Molecules 2021, 26, 6840. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Rong, W.; Adu-Frimpong, M.; He, Q.; Li, X.; Shi, F.; Ji, H.; Toreniyazov, E.; Xia, X.; Zhang, J.; et al. Preparation, in vitro and in vivo evaluation of pinocembrin-loaded TPGS modified liposomes with enhanced bioavailability and antihyperglycemic activity. Drug Dev. Ind. Pharm. 2022, 48, 623–634. [Google Scholar] [CrossRef]
- Trapani, A.; Guerra, L.; Corbo, F.; Castellani, S.; Sanna, E.; Capobianco, L.; Monteduro, A.G.; Manno, D.E.; Mandracchia, D.; Di Gioia, S.; et al. Cyto/Biocompatibility of Dopamine Combined with the Antioxidant Grape Seed-Derived Polyphenol Compounds in Solid Lipid Nanoparticles. Molecules 2021, 26, 916. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, R.; Liu, L.; Chi, J.; Huang, F.; Dong, L.; Ma, Q.; Jia, X.; Zhang, M. Preparation, stability and antioxidant capacity of nano liposomes loaded with procyandins from lychee pericarp. J. Food Eng. 2020, 284, 110065. [Google Scholar] [CrossRef]
- Aly, S.; El-Kamel, A.H.; Sheta, E.; El-Habashy, S.E. Chondroitin/Lactoferrin-dual functionalized pterostilbene-solid lipid nanoparticles as targeted breast cancer therapy. Int. J. Pharm. 2023, 642, 123163. [Google Scholar] [CrossRef]
- Hosseinpour Jajarm, F.; Moravvej, G.; Modarres Awal, M.; Golmohammadzadeh, S. Insecticidal activity of solid lipid nanoparticle loaded by Ziziphora clinopodioides Lam. against Tribolium castaneum (Herbst, 1797) (Coleoptera: Tenebrionidae). Int. J. Pest Manag. 2021, 67, 147–154. [Google Scholar] [CrossRef]
- Mahadev, M.; Nandini, H.S.; Ramu, R.; Gowda, D.V.; Almarhoon, Z.M.; Al-Ghorbani, M.; Mabkhot, Y.N. Fabrication and Evaluation of Quercetin Nanoemulsion: A Delivery System with Improved Bioavailability and Therapeutic Efficacy in Diabetes Mellitus. Pharmaceuticals 2022, 15, 70. [Google Scholar] [CrossRef]
- Saatkamp, R.H.; Sanches, M.P.; Gambin, J.P.D.; Amaral, B.R.; Farias, N.S.d.; Caon, T.; Müller, C.M.O.; Parize, A.L. Development of thymol nanoemulsions with potential application in oral infections. J. Drug Deliv. Sci. Technol. 2023, 87, 104855. [Google Scholar] [CrossRef]
- Alkhatib, M.H.; Bawadud, R.S.; Gashlan, H.M. Incorporation of docetaxel and thymoquinone in borage nanoemulsion potentiates their antineoplastic activity in breast cancer cells. Sci. Rep. 2020, 10, 18124. [Google Scholar] [CrossRef] [PubMed]
- Abeesh, P.; Guruvayoorappan, C. Umbelliferone loaded PEGylated liposomes: Preparation, characterization and its mitigatory effects on Dalton’s ascites lymphoma development. 3 Biotech 2023, 13, 216. [Google Scholar] [CrossRef]
- Stahl, W.; Heinrich, U.; Aust, O.; Tronnier, H.; Sies, H. Lycopene-rich products and dietary photoprotection. Photochem. Photobiol. Sci. 2006, 5, 238–242. [Google Scholar] [CrossRef]
- Shi, J.; Maguer, M.; Bryan, M.; Kakuda, Y. Kinetics of lycopene degradation in tomato puree by heat and light irradiation. J. Food Process Eng. 2003, 25, 485–498. [Google Scholar] [CrossRef]
- Sharma, S.K.; Le Maguer, M. Kinetics of lycopene degradation in tomato pulp solids under different processing and storage conditions. Food Res. Int. 1996, 29, 309–315. [Google Scholar] [CrossRef]
- Riangjanapatee, P.; Okonogi, S. Effect of surfactant on lycopene-loaded nanostructured lipid carriers. Drug Discov. Ther. 2012, 6, 163–168. [Google Scholar] [CrossRef]
- Riangjanapatee, P.; Müller, R.; Keck, C.; Okonogi, S. Development of lycopene-loaded nanostructured lipid carriers: Effect of rice oil and cholesterol. Die Pharm.-Int. J. Pharm. Sci. 2013, 68, 723–731. [Google Scholar]
- Okonogi, S.; Riangjanapatee, P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int. J. Pharm. 2015, 478, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Akhoond Zardini, A.; Mohebbi, M.; Farhoosh, R.; Bolurian, S. Production and characterization of nanostructured lipid carriers and solid lipid nanoparticles containing lycopene for food fortification. J. Food Sci. Technol. 2018, 55, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xin, Z.; Li, N.; Chang, S.; Chen, Y.; Geng, L.; Chang, H.; Shi, H.; Chang, Y.-Z. Nano-liposomes of lycopene reduces ischemic brain damage in rodents by regulating iron metabolism. Free Radic. Biol. Med. 2018, 124, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, N.; Ilic, S.; Jakovljevic, V.; Stojanovic, N.; Stojnev, S.; Kocic, H.; Stojanovic, M.; Kocic, G. The encapsulation of lycopene in nanoliposomes enhances its protective potential in methotrexate-induced kidney injury model. Oxidative Med. Cell. Longev. 2018, 2018, 2627917. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, Q.; Shen, S. Enhanced antitumor efficacy and attenuated cardiotoxicity of doxorubicin in combination with lycopene liposomes. J. Liposome Res. 2020, 30, 37–44. [Google Scholar] [CrossRef]
- Li, D.; Li, L.; Xiao, N.; Li, M.; Xie, X. Physical properties of oil-in-water nanoemulsions stabilized by OSA-modified starch for the encapsulation of lycopene. Colloids Surf. Physicochem. Eng. Asp. 2018, 552, 59–66. [Google Scholar] [CrossRef]
- Zhao, C.; Wei, L.; Yin, B.; Liu, F.; Li, J.; Liu, X.; Wang, J.; Wang, Y. Encapsulation of lycopene within oil-in-water nanoemulsions using lactoferrin: Impact of carrier oils on physicochemical stability and bioaccessibility. Int. J. Biol. Macromol. 2020, 153, 912–920. [Google Scholar] [CrossRef]
- Altincicek, B.; Kovacs, J.L.; Gerardo, N.M. Horizontally transferred fungal carotenoid genes in the two-spotted spider mite Tetranychus urticae. Biol. Lett. 2012, 8, 253–257. [Google Scholar] [CrossRef]
- Armstrong, G.A.; Hearst, J.E. Carotenoids 2: Genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J. 1996, 10, 228–237. [Google Scholar] [CrossRef]
- Bendich, A.; Olson, J.A. Biological actions of carotenoids. FASEB J. 1989, 3, 1927–1932. [Google Scholar] [CrossRef]
- Horn, D.; Lüddecke, E. Preparation and characterization of nano-sized carotenoid hydrosols. In Fine Particles Science and Technology; Springer: Berlin/Heidelberg, Germany, 1996; pp. 761–775. [Google Scholar]
- Hentschel, A.; Gramdorf, S.; Müller, R.; Kurz, T. β-Carotene-loaded nanostructured lipid carriers. J. Food Sci. 2008, 73, N1–N6. [Google Scholar] [CrossRef]
- Ryan, L.; O’Connell, O.; O’Sullivan, L.; Aherne, S.; O’Brien, N. Micellarisation of carotenoids from raw and cooked vegetables. Plant Foods Hum. Nutr. 2008, 63, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Helgason, T.; Awad, T.S.; Kristbergsson, K.; Decker, E.A.; McClements, D.J.; Weiss, J. Impact of surfactant properties on oxidative stability of β-carotene encapsulated within solid lipid nanoparticles. J. Agric. Food Chem. 2009, 57, 8033–8040. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Cellular uptake of β-carotene from protein stabilized solid lipid nanoparticles prepared by homogenization–evaporation method. J. Agric. Food Chem. 2014, 62, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Escobar, M.; Pascual-Mathey, L.; Beristain, C.; Flores-Andrade, E.; Jiménez, M.; Pascual-Pineda, L. In vitro and In vivo antioxidant properties of paprika carotenoids nanoemulsions. LWT 2020, 118, 108694. [Google Scholar] [CrossRef]
- Flores-Andrade, E.; Allende-Baltazar, Z.; Sandoval-González, P.E.; Jiménez-Fernández, M.; Beristain, C.I.; Pascual-Pineda, L.A. Carotenoid nanoemulsions stabilized by natural emulsifiers: Whey protein, gum Arabic, and soy lecithin. J. Food Eng. 2021, 290, 110208. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Chen, B.-H. A comparative study on inhibition of breast cancer cells and tumors in mice by carotenoid extract and nanoemulsion prepared from sweet potato (Ipomoea batatas L.) peel. Pharmaceutics 2022, 14, 980. [Google Scholar] [CrossRef]
- Chami, F.; Chami, N.; Bennis, S.; Trouillas, J.; Remmal, A. Evaluation of carvacrol and eugenol as prophylaxis and treatment of vaginal candidiasis in an immunosuppressed rat model. J. Antimicrob. Chemother. 2004, 54, 909–914. [Google Scholar] [CrossRef]
- He, M.; Du, M.; Fan, M.; Bian, Z. In vitro activity of eugenol against Candida albicans biofilms. Mycopathologia 2007, 163, 137–143. [Google Scholar] [CrossRef]
- Garg, A.; Singh, S. Enhancement in antifungal activity of eugenol in immunosuppressed rats through lipid nanocarriers. Colloids Surf. B Biointerfaces 2011, 87, 280–288. [Google Scholar] [CrossRef]
- Pokharkar, V.B.; Shekhawat, P.B.; Dhapte, V.V.; Mandpe, L.P. Development and optimization of eugenol loaded nanostructured lipid carriers for periodontal delivery. Int. J. Pharm. Pharm. Sci. 2011, 3, 138–143. [Google Scholar]
- Garg, A.; Singh, S. Targeting of eugenol-loaded solid lipid nanoparticles to the epidermal layer of human skin. Nanomedicine 2014, 9, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Rodenak-Kladniew, B.; Montoto, S.S.; Sbaraglini, M.L.; Di Ianni, M.; Ruiz, M.E.; Talevi, A.; Alvarez, V.A.; Durán, N.; Castro, G.R.; Islan, G.A. Hybrid Ofloxacin/eugenol co-loaded solid lipid nanoparticles with enhanced and targetable antimicrobial properties. Int. J. Pharm. 2019, 569, 118575. [Google Scholar] [CrossRef] [PubMed]
- Mishra, H.; Mishra, P.K.; Iqbal, Z.; Jaggi, M.; Madaan, A.; Bhuyan, K.; Gupta, N.; Gupta, N.; Vats, K.; Verma, R. Co-delivery of eugenol and dacarbazine by hyaluronic acid-coated liposomes for targeted inhibition of survivin in treatment of resistant metastatic melanoma. Pharmaceutics 2019, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, X.; Zhang, T.; Zhang, L.; Yang, J.; Li, Y.; Shen, J.; Wang, J.; Niu, Y.; Xiao, Z. Cationic and temperature-sensitive liposomes loaded with eugenol for the application to silk. Chin. Chem. Lett. 2020, 31, 3139–3142. [Google Scholar] [CrossRef]
- Revi, N.; Sankaranarayanan, S.A.; Rengan, A.K. A study on the role of eugenol encapsulated liposomes in facilitating neuron-microglia mediated wound recovery. Materialia 2022, 23, 101454. [Google Scholar] [CrossRef]
- Sebaaly, C.; Haydar, S.; Greige-Gerges, H. Eugenol encapsulation into conventional liposomes and chitosan-coated liposomes: A comparative study. J. Drug Deliv. Sci. Technol. 2022, 67, 102942. [Google Scholar] [CrossRef]
- Tanzeem, M.U.; Asghar, S.; Khalid, S.H.; Asif, M.; Ullah, M.S.; Khan, I.U.; Khalid, I.; Faran, S.A.; Rehman, A.; Gohar, U.F. Clove oil based co-surfactant free microemulsion of flurbiprofen: Improved solubility with ameliorated drug-induced gastritis. Pak. J. Pharm. Sci. 2019, 32, 2787. [Google Scholar]
- Khanna, N. Turmeric-Nature’s precious gift. Curr. Sci. 1999, 76, 1351–1356. [Google Scholar]
- Rainey, N.E.; Moustapha, A.; Saric, A.; Nicolas, G.; Sureau, F.; Petit, P.X. Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation. Cell Death Discov. 2019, 5, 150. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Tiyaboonchai, W.; Tungpradit, W.; Plianbangchang, P. Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int. J. Pharm. 2007, 337, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Plianbangchang, P.; Tungpradit, W.; Tiyaboonchai, W. Efficacy and safety of curcuminoids loaded solid lipid nanoparticles facial cream as an anti-aging agent. Naresuan Univ. J. 2007, 15, 73–81. [Google Scholar]
- Kakkar, V.; Singh, S.; Singla, D.; Kaur, I.P. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol. Nutr. Food Res. 2011, 55, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.; Kaur, I.P. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem. Toxicol. 2011, 49, 2906–2913. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.; Bhushan, S.; Guru, S.K.; Kaur, I.P. Enhanced apoptotic effect of curcumin loaded solid lipid nanoparticles. Mol. Pharm. 2012, 9, 3411–3421. [Google Scholar]
- Kakkar, V.; Muppu, S.K.; Chopra, K.; Kaur, I.P. Curcumin loaded solid lipid nanoparticles: An efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur. J. Pharm. Biopharm. 2013, 85, 339–345. [Google Scholar] [CrossRef]
- Balasubramanyam, K.; Varier, R.A.; Altaf, M.; Swaminathan, V.; Siddappa, N.B.; Ranga, U.; Kundu, T.K. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J. Biol. Chem. 2004, 279, 51163–51171. [Google Scholar] [CrossRef]
- Puglia, C.; Frasca, G.; Musumeci, T.; Rizza, L.; Puglisi, G.; Bonina, F.; Chiechio, S. Curcumin loaded NLC induces histone hypoacetylation in the CNS after intraperitoneal administration in mice. Eur. J. Pharm. Biopharm. 2012, 81, 288–293. [Google Scholar] [CrossRef]
- Meng, F.; Asghar, S.; Xu, Y.; Wang, J.; Jin, X.; Wang, Z.; Wang, J.; Ping, Q.; Zhou, J.; Xiao, Y. Design and evaluation of lipoprotein resembling curcumin-encapsulated protein-free nanostructured lipid carrier for brain targeting. Int. J. Pharm. 2016, 506, 46–56. [Google Scholar] [CrossRef]
- Meng, F.; Asghar, S.; Gao, S.; Su, Z.; Song, J.; Huo, M.; Meng, W.; Ping, Q.; Xiao, Y. A novel LDL-mimic nanocarrier for the targeted delivery of curcumin into the brain to treat Alzheimer's disease. Colloids Surf. B Biointerfaces 2015, 134, 88–97. [Google Scholar] [CrossRef]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W.; Kweon, D.-H.; Choi, Y.J. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef]
- Singh, S.P.; Alvi, S.B.; Pemmaraju, D.B.; Singh, A.D.; Manda, S.V.; Srivastava, R.; Rengan, A.K. NIR triggered liposome gold nanoparticles entrapping curcumin as in situ adjuvant for photothermal treatment of skin cancer. Int. J. Biol. Macromol. 2018, 110, 375–382. [Google Scholar] [CrossRef]
- Wu, Y.; Mou, B.; Song, S.; Tan, C.-P.; Lai, O.-M.; Shen, C.; Cheong, L.-Z. Curcumin-loaded liposomes prepared from bovine milk and krill phospholipids: Effects of chemical composition on storage stability, in-vitro digestibility and anti-hyperglycemic properties. Food Res. Int. 2020, 136, 109301. [Google Scholar] [CrossRef]
- Bhatia, E.; Sharma, S.; Jadhav, K.; Banerjee, R. Combinatorial liposomes of berberine and curcumin inhibit biofilm formation and intracellular methicillin resistant Staphylococcus aureus infections and associated inflammation. J. Mater. Chem. B 2021, 9, 864–875. [Google Scholar] [CrossRef]
- Song, J.-W.; Liu, Y.-S.; Guo, Y.-R.; Zhong, W.-X.; Guo, Y.-P.; Guo, L. Nano–liposomes double loaded with curcumin and tetrandrine: Preparation, characterization, hepatotoxicity and anti–tumor effects. Int. J. Mol. Sci. 2022, 23, 6858. [Google Scholar] [CrossRef]
- Páez-Hernández, G.; Mondragón-Cortez, P.; Espinosa-Andrews, H. Developing curcumin nanoemulsions by high-intensity methods: Impact of ultrasonication and microfluidization parameters. LWT 2019, 111, 291–300. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Al-Qudaihi, A.; Alaseel, S.E.; Fita, I.Z.; Khalid, M.S.; Pottoo, F.H. Preparation of a novel curcumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation. RSC Adv. 2019, 9, 20192–20206. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Prasongsuk, S.; Sabliov, C.M. Effect of surfactant concentrations on physicochemical properties and functionality of curcumin nanoemulsions under conditions relevant to commercial utilization. Molecules 2019, 24, 2744. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Peng, H.; Li, Y.; Xiong, J.; Xu, Z. The formulation and delivery of curcumin with solid lipid nanoparticles for the treatment of on non-small cell lung cancer both in vitro and in vivo. Mater. Sci. Eng. C 2013, 33, 4802–4808. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Q.; Liu, W.; Li, Y.; Tang, H.; Liu, X.; Yang, X. Codelivery of doxorubicin and curcumin with lipid nanoparticles results in improved efficacy of chemotherapy in liver cancer. Int. J. Nanomed. 2015, 10, 257. [Google Scholar]
- Chirio, D.; Gallarate, M.; Peira, E.; Battaglia, L.; Serpe, L.; Trotta, M. Formulation of curcumin-loaded solid lipid nanoparticles produced by fatty acids coacervation technique. J. Microencaps. 2011, 28, 537–548. [Google Scholar] [CrossRef]
- Sun, J.; Bi, C.; Chan, H.M.; Sun, S.; Zhang, Q.; Zheng, Y. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids Surf. B Biointerfaces 2013, 111, 367–375. [Google Scholar] [CrossRef]
- Mulik, R.S.; Mönkkönen, J.; Juvonen, R.O.; Mahadik, K.R.; Paradkar, A.R. Transferrin mediated solid lipid nanoparticles containing curcumin: Enhanced in vitro anticancer activity by induction of apoptosis. Int. J. Pharm. 2010, 398, 190–203. [Google Scholar] [CrossRef]
- Xu, H.; Gong, Z.; Zhou, S.; Yang, S.; Wang, D.; Chen, X.; Wu, J.; Liu, L.; Zhong, S.; Zhao, J. Liposomal curcumin targeting endometrial Cancer through the NF-κB pathway. Cell. Physiol. Biochem. 2018, 48, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, B.A.; Reddy, A.S.; Sangubotla, R.; Hong, J.W.; Kim, S. Ruthenium (II)-curcumin liposome nanoparticles: Synthesis, characterization, and their effects against cervical cancer. Colloids Surf. B Biointerfaces 2021, 204, 111773. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.; Sheashaa, R.F.; Moussa, E.A.; Mahfouz, M.E. Potential of curcumin and niacin-loaded targeted chitosan coated liposomes to activate autophagy in hepatocellular carcinoma cells: An in vitro evaluation in HePG2 cell line. Int. J. Biol. Macromol. 2023, 245, 125572. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.K.; El Kurdi, R.; Badran, A.; Mesmar, J.; Baydoun, E.; Patra, D. Liposome-based nanocapsules for the controlled release of dietary curcumin: PDDA and silica nanoparticle-coated DMPC liposomes enhance the fluorescence efficiency and anticancer activity of curcumin. RSC Adv. 2022, 12, 11282–11292. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.C.; de Matos, R.P.A.; Primo, F.L.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Effect of curcumin-nanoemulsion associated with photodynamic therapy in breast adenocarcinoma cell line. Biorg. Med. Chem. 2019, 27, 1882–1890. [Google Scholar] [CrossRef]
- Li, K.; Pi, C.; Wen, J.; He, Y.; Yuan, J.; Shen, H.; Zhao, W.; Zeng, M.; Song, X.; Lee, R.J. Formulation of the novel structure curcumin derivative–loaded solid lipid nanoparticles: Synthesis, optimization, characterization and anti-tumor activity screening in vitro. Drug Deliv. 2022, 29, 2044–2057. [Google Scholar] [CrossRef]
- Fathy Abd-Ellatef, G.-E.; Gazzano, E.; Chirio, D.; Ragab Hamed, A.; Belisario, D.C.; Zuddas, C.; Peira, E.; Rolando, B.; Kopecka, J.; Assem Said Marie, M. Curcumin-loaded solid lipid nanoparticles bypass p-glycoprotein mediated doxorubicin resistance in triple negative breast cancer cells. Pharmaceutics 2020, 12, 96. [Google Scholar] [CrossRef]
- Guo, P.; Pi, C.; Zhao, S.; Fu, S.; Yang, H.; Zheng, X.; Zhang, X.; Zhao, L.; Wei, Y. Oral co-delivery nanoemulsion of 5-fluorouracil and curcumin for synergistic effects against liver cancer. Expert Opin. Drug Deliv. 2020, 17, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Youssry, S.A.; El-Sheredy, H.G.; Shalaby, T.I. In vitro evaluation of antitumor and immunomodulatory potential of curcumin nano-emulsion on breast cancer. BioNanoScience 2022, 12, 841–850. [Google Scholar] [CrossRef]
- Angeline, N.; Suhito, I.R.; Kim, C.-H.; Hong, G.-P.; Park, C.G.; Bhang, S.H.; Luo, Z.; Kim, T.-H. A fibronectin-coated gold nanostructure composite for electrochemical detection of effects of curcumin-carrying nanoliposomes on human stomach cancer cells. Analyst 2020, 145, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Sheikhpour, M.; Sadeghizadeh, M.; Yazdian, F.; Mansoori, A.; Asadi, H.; Movafagh, A.; Shahraeini, S.S. Co-administration of curcumin and bromocriptine nano-liposomes for induction of apoptosis in lung cancer cells. Iran. Biomed. J. 2020, 24, 24. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wei, Y.; Sheng, L.; Ma, J.; Su, Z.; Wen, J.; Li, L.; Jia, Q.; Liu, H.; Si, H. Construction of Curcumin and Paclitaxel Co-Loaded Lipid Nano Platform and Evaluation of Its Anti-Hepatoma Activity in vitro and Pharmacokinetics in vivo. Int. J. Nanomed. 2023, 18, 2087–2107. [Google Scholar] [CrossRef]
- Wu, C.; Yang, J.; Wang, F.; Wang, X. Resveratrol: Botanical origin, pharmacological activity and applications. Chin. J. Nat. Med. 2013, 11, 1–15. [Google Scholar] [CrossRef]
- Ge, J.-F.; Qiao, J.-P.; Qi, C.-C.; Wang, C.-W.; Zhou, J.-N. The binding of resveratrol to monomer and fibril amyloid beta. Neurochem. Int. 2012, 61, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Amri, A.; Chaumeil, J.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef]
- Pandita, D.; Kumar, S.; Poonia, N.; Lather, V. Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res. Int. 2014, 62, 1165–1174. [Google Scholar] [CrossRef]
- Neves, A.R.; Martins, S.; Segundo, M.A.; Reis, S. Nanoscale delivery of resveratrol towards enhancement of supplements and nutraceuticals. Nutrients 2016, 8, 131. [Google Scholar] [CrossRef]
- Neves, A.R.; Queiroz, J.F.; Reis, S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J. Nanobiotechnol. 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Ko, Y.T. Enhanced Subcellular Trafficking of Resveratrol Using Mitochondriotropic Liposomes in Cancer Cells. Pharmaceutics 2019, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Clifford, M.N. Flavanones, chalcones and dihydrochalcones–nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1073–1080. [Google Scholar] [CrossRef]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Sansone, F.; Rossi, A.; Del Gaudio, P.; De Simone, F.; Aquino, R.P.; Lauro, M.R. Hesperidin gastroresistant microparticles by spray-drying: Preparation, characterization, and dissolution profiles. AAPS PharmSciTech 2009, 10, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Varshosaz, J.; Mohebbi, M.; Shahidi, F. hesperetin-loaded solid lipid nanoparticles and nanostructure lipid carriers for food fortification: Preparation, characterization, and modeling. Food Bioprocess Technol. 2013, 6, 1464–1475. [Google Scholar] [CrossRef]
- Zeng, F.; Wang, D.; Tian, Y.; Wang, M.; Liu, R.; Xia, Z.; Huang, Y. Nanoemulsion for Improving the Oral Bioavailability of Hesperetin: Formulation Optimization and Absorption Mechanism. J. Pharm. Sci. 2021, 110, 2555–2561. [Google Scholar] [CrossRef]
- Wang, X.; Song, Y.; Yu, L.; Xue, X.; Pang, M.; Li, Y.; Luo, X.; Hua, Z.; Lu, C.; Lu, A. Co-Delivery of Hesperetin and Cisplatin via Hyaluronic Acid-Modified Liposome for Targeted Inhibition of Aggression and Metastasis of Triple-Negative Breast Cancer. ACS Appl. Mater. Interfaces 2023, 15, 29. [Google Scholar] [CrossRef]
- Clozel, J.; Roberts, A.; Hoffman, J.; Coleridge, H.; Coleridge, J. Vagal chemoreflex coronary vasodilation evoked by stimulating pulmonary C-fibers in dogs. Circ. Res. 1985, 57, 450–460. [Google Scholar] [CrossRef]
- Fusco, B.M.; Giacovazzo, M. Peppers and pain. Drugs 1997, 53, 909–914. [Google Scholar] [CrossRef]
- Sharma, A.; Arora, S. Development of Topical Gel of Capsaicin Loaded Solid Lipid Nanoparticles (SLNs): In vitro and in vivo Evaluation. Indian J. Pharm. 2011, 2, 29–41. [Google Scholar]
- Duangjit, S.; Pucharean, C.; Yoddee, P.; Kanlayawuttipong, K.; Ngawhirunpat, T. Effect of Terpene on Physicochemical Properties and Skin Permeability of Capsaicin Loaded Solid Lipid Nanoparticles. Isan J. Pharm. Sci. 2016, 11, 124–134. [Google Scholar]
- Kunjiappan, S.; Sankaranarayanan, M.; Kumar, B.K.; Pavadai, P.; Babkiewicz, E.; Maszczyk, P.; Glodkowska-Mrowka, E.; Arunachalam, S.; Pandian, S.R.K.; Ravishankar, V. Capsaicin-loaded solid lipid nanoparticles: Design, biodistribution, in silico modeling and in vitro cytotoxicity evaluation. Nanotechnology 2020, 32, 095101. [Google Scholar] [CrossRef]
- Stark, T.; Bareuther, S.; Hofmann, T. Sensory-guided decomposition of roasted cocoa nibs (Theobroma cacao) and structure determination of taste-active polyphenols. J. Agric. Food Chem. 2005, 53, 5407–5418. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-C.; Ko, C.H.; Tseng, S.-W.; Tsai, S.-H.; Chen, Y.-C. Structurally related antitumor effects of flavanones in vitro and in vivo: Involvement of caspase 3 activation, p21 gene expression, and reactive oxygen species production. Toxicol. Appl. Pharmacol. 2004, 197, 84–95. [Google Scholar] [CrossRef]
- Kanno, S.-I.; Tomizawa, A.; Hiura, T.; Osanai, Y.; Shouji, A.; Ujibe, M.; Ohtake, T.; Kimura, K.; Ishikawa, M. Inhibitory effects of naringenin on tumor growth in human cancer cell lines and sarcoma S-180-implanted mice. Biol. Pharm. Bull. 2005, 28, 527–530. [Google Scholar] [CrossRef]
- Ratnam, D.V.; Ankola, D.; Bhardwaj, V.; Sahana, D.; Kumar, M.R. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control. Release 2006, 113, 189–207. [Google Scholar] [CrossRef]
- Ji, P.; Yu, T.; Liu, Y.; Jiang, J.; Xu, J.; Zhao, Y.; Hao, Y.; Qiu, Y.; Zhao, W.; Wu, C. naringenin-loaded solid lipid nanoparticles: Preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des. Dev. Ther. 2016, 10, 911. [Google Scholar]
- Wang, L.; Wang, X.; Shen, L.; Alrobaian, M.; Panda, S.K.; Almasmoum, H.A.; Ghaith, M.M.; Almaimani, R.A.; Ibrahim, I.A.A.; Singh, T. Paclitaxel and naringenin-loaded solid lipid nanoparticles surface modified with cyclic peptides with improved tumor targeting ability in glioblastoma multiforme. Biomed. Pharmacother. 2021, 138, 111461. [Google Scholar] [CrossRef]
- Munir, A.; Muhammad, F.; Zaheer, Y.; Ali, M.A.; Iqbal, M.; Rehman, M.; Munir, M.U.; Akhtar, B.; Webster, T.J.; Sharif, A. Synthesis of naringenin loaded lipid based nanocarriers and their in-vivo therapeutic potential in a rheumatoid arthritis model. J. Drug Deliv. Sci. Technol. 2021, 66, 102854. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Yin, W.; Liu, Z.; Shi, L.; Tang, M. A novel drug delivery system: The encapsulation of naringenin in metal-organic frameworks into liposomes. AAPS PharmSciTech 2021, 22, 61. [Google Scholar] [CrossRef]
- Kong, F.; Luan, Y.; Zhang, Z.H.; Cheng, G.H.; Qi, T.G.; Sun, C. Baicalin protects the myocardium from reperfusion-induced damage in isolated rat hearts via the antioxidant and paracrine effect. Exp. Ther. Med. 2014, 7, 254–259. [Google Scholar] [CrossRef]
- Tu, X.-K.; Yang, W.-Z.; Shi, S.-S.; Wang, C.-H.; Chen, C.-M. Neuroprotective effect of baicalin in a rat model of permanent focal cerebral ischemia. Neurochem. Res. 2009, 34, 1626–1634. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; He, Q.; Liu, X.; Ikechukwu, O.C.; Tong, L.; Guo, L.; Yang, H.; Zhang, Q.; Zhao, H. Effect of Baicalin-loaded PEGylated cationic solid lipid nanoparticles modified by OX26 antibody on regulating the levels of baicalin and amino acids during cerebral ischemia–reperfusion in rats. Int. J. Pharm. 2015, 489, 131–138. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Wan, J.; Yang, Q.; Xiang, Y.; Ni, L.; Long, Y.; Cui, M.; Ci, Z.; Tang, D.; et al. Preparation, Characterization and in vivo Study of Borneol-Baicalin-Liposomes for Treatment of Cerebral Ischemia-Reperfusion Injury. Int. J. Nanomed. 2020, 15, 5977–5989. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, A.; Wu, H.; Bi, Y. Development and in vivo evaluation of baicalin-loaded W/O nanoemulsion for lymphatic absorption. Pharm. Dev. Technol. 2019, 24, 1155–1163. [Google Scholar] [CrossRef]
- Lindsey, K.; Pullen, M.L.; Topping, J.F. Importance of plant sterols in pattern formation and hormone signalling. Trends Plant Sci. 2003, 8, 521–525. [Google Scholar] [CrossRef]
- Ostlund, R.E., Jr. Phytosterols in human nutrition. Annu. Rev. Nutr. 2002, 22, 533–549. [Google Scholar] [CrossRef]
- Racette, S.B.; Lin, X.; Lefevre, M.; Spearie, C.A.; Most, M.M.; Ma, L.; Ostlund, R.E. Dose effects of dietary phytosterols on cholesterol metabolism: A controlled feeding study. Am. J. Clin. Nutr. 2010, 91, 32–38. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, N.; Stan, R.; Meghea, A. Novel bio-active lipid nanocarriers for the stabilization and sustained release of sitosterol. Nanotechnology 2012, 23, 455702. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.; He, X.; Li, Z.; Shi, B.; Cai, F. β-Sitosterol-loaded solid lipid nanoparticles ameliorate complete Freund’s adjuvant-induced arthritis in rats: Involvement of NF-кB and HO-1/Nrf-2 pathway. Drug Deliv. 2020, 27, 1329–1341. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-Y.; Lee, C.-F.; Chou, W.-T.; Hwang, J.-J.; Tyan, Y.-S.; Chuang, H.-Y. Liposomal β-Sitosterol Suppresses Metastasis of CT26/luc Colon Carcinoma via Inhibition of MMP-9 and Evoke of Immune System. Pharmaceutics 2022, 14, 1214. [Google Scholar] [CrossRef] [PubMed]
- Chennuru, A.; Saleem, M.T. Antioxidant, lipid lowering, and membrane stabilization effect of sesamol against doxorubicin-induced cardiomyopathy in experimental rats. BioMed Res. Int. 2013, 2013, 934239. [Google Scholar] [CrossRef]
- Geetha, T.; Singh, N.; Deol, P.K.; Kaur, I.P. Biopharmaceutical profiling of sesamol: Physiochemical characterization, gastrointestinal permeability and pharmacokinetic evaluation. RSC Adv. 2015, 5, 4083–4091. [Google Scholar] [CrossRef]
- Wenzel, E.; Soldo, T.; Erbersdobler, H.; Somoza, V. Bioactivity and metabolism of trans-resveratrol orally administered to Wistar rats. Mol. Nutr. Food Res. 2005, 49, 482–494. [Google Scholar] [CrossRef]
- Jan, K.C.; Ho, C.T.; Hwang, L.S. Elimination and metabolism of sesamol, a bioactive compound in sesame oil, in rats. Mol. Nutr. Food Res. 2009, 53, S36–S43. [Google Scholar] [CrossRef]
- VanGilder, R.; Kelly, K.; Chua, M.; Ptachcinski, R.; Huber, J.D. Administration of sesamol improved blood–brain barrier function in streptozotocin-induced diabetic rats. Exp. Brain Res. 2009, 197, 23–34. [Google Scholar] [CrossRef]
- Geetha, T.; Rohit, B.; Pal, K.I. Sesamol: An efficient antioxidant with potential therapeutic benefits. Med. Chem. 2009, 5, 367–371. [Google Scholar] [CrossRef]
- Kakkar, V.; Mishra, A.K.; Chuttani, K.; Chopra, K.; Kaur, I.P. Delivery of sesamol-loaded solid lipid nanoparticles to the brain for menopause-related emotional and cognitive central nervous system derangements. Rejuvenation Res. 2011, 14, 597–604. [Google Scholar] [CrossRef]
- Morisco, F.; Vitaglione, P.; Amoruso, D.; Russo, B.; Fogliano, V.; Caporaso, N. Foods and liver health. Mol. Asp. Med. 2008, 29, 144–150. [Google Scholar] [CrossRef]
- Kumagai, Y.; Lin, L.Y.; Schmitz, D.A.; Cho, A.K. Hydroxyl radical mediated demethylenation of (methylenedioxy)phenyl compounds. Chem. Res. Toxicol. 1991, 4, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Khullar, N.; Kakkar, V.; Kaur, I.P. Hepatoprotective effects of sesamol loaded solid lipid nanoparticles in carbon tetrachloride induced sub-chronic hepatotoxicity in rats. Environ. Toxicol. 2014, 31, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Khullar, N.; Kakkar, V.; Kaur, I.P. Sesamol loaded solid lipid nanoparticles: A promising intervention for control of carbon tetrachloride induced hepatotoxicity. BMC Complement. Altern. Med. 2015, 15, 142. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.K.; Misra, S.; Kaur, I.P.; Chopra, K. Neuroprotective potential of sesamol and its loaded solid lipid nanoparticles in ICV-STZ-induced cognitive deficits: Behavioral and biochemical evidence. Eur. J. Pharmacol. 2015, 747, 132–140. [Google Scholar] [CrossRef]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- Ames, B.N.; Durston, W.E.; Yamasaki, E.; Lee, F.D. Carcinogens are mutagens: A simple test system combining liver homogenates for activation and bacteria for detection. Proc. Natl. Acad. Sci. USA 1973, 70, 2281–2285. [Google Scholar] [CrossRef]
- Geetha, T.; Kapila, M.; Prakash, O.; Deol, P.K.; Kakkar, V.; Kaur, I.P. Sesamol-loaded solid lipid nanoparticles for treatment of skin cancer. J. Drug Target. 2015, 23, 159–169. [Google Scholar] [CrossRef]
- Yousefi, S.; Rajaei, P.; Nateghi, L.; Nodeh, H.R.; Rashidi, L. Encapsulation of sesamol and retinol using alginate and chitosan-coated W/O/W multiple emulsions containing Tween 80 and Span 80. Int. J. Biol. Macromol. 2023, 242, 124766. [Google Scholar] [CrossRef]
- Sutaria, D.; Grandhi, B.K.; Thakkar, A.; Wang, J.; Prabhu, S. Chemoprevention of pancreatic cancer using solid-lipid nanoparticulate delivery of a novel aspirin, curcumin and sulforaphane drug combination regimen. Int. J. Oncol. 2012, 41, 2260–2268. [Google Scholar] [CrossRef]
- Thakkar, A.; Chenreddy, S.; Wang, J.; Prabhu, S. Preclinical systemic toxicity evaluation of chitosan-solid lipid nanoparticles encapsulated aspirin, curcumin and free sulforaphane (ACS) combinations in BALB/c mice. Cancer Res. 2015, 75, 2621. [Google Scholar] [CrossRef]
- Abdelwahab, S.I.; Sheikh, B.Y.; Taha, M.M.E.; How, C.W.; Abdullah, R.; Yagoub, U.; El-Sunousi, R.; Eid, E. Thymoquinone-loaded nanostructured lipid carriers: Preparation, gastroprotection, in vitro toxicity, and pharmacokinetic properties after extravascular administration. Int. J. Nanomed. 2013, 8, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.A.; Madureira, A.R.; Gomes, A.M.; Sarmento, B.; Pintado, M.M. Optimization of the production of solid Witepsol nanoparticles loaded with rosmarinic acid. Colloids Surf. B Biointerfaces 2014, 115, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Madureira, A.R.; Campos, D.A.; Fonte, P.; Nunes, S.; Reis, F.; Gomes, A.M.; Sarmento, B.; Pintado, M.M. Characterization of solid lipid nanoparticles produced with carnauba wax for rosmarinic acid oral delivery. RSC Adv. 2015, 5, 22665–22673. [Google Scholar] [CrossRef]
- Bhatt, R.; Singh, D.; Prakash, A.; Mishra, N. Development, characterization and nasal delivery of rosmarinic acid-loaded solid lipid nanoparticles for the effective management of Huntington’s disease. Drug Deliv. 2015, 22, 931–939. [Google Scholar] [CrossRef]
- Li, M.; Zahi, M.R.; Yuan, Q.; Tian, F.; Liang, H. Preparation and stability of astaxanthin solid lipid nanoparticles based on stearic acid. Eur. J. Lipid Sci. Technol. 2015, 118, 592–602. [Google Scholar] [CrossRef]
- Bhatt, P.C.; Srivastava, P.; Pandey, P.; Khan, W.; Panda, B.P. Nose to brain delivery of astaxanthin-loaded solid lipid nanoparticles: Fabrication, radio labeling, optimization and biological studies. RSC Adv. 2016, 6, 10001–10010. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Andreani, T.; Fernandes, L.; Garcia, M.L.; Egea, M.A.; Silva, A.M.; Souto, E.B. Physicochemical characterization of epigallocatechin gallate lipid nanoparticles (EGCG-LNs) for ocular instillation. Colloids Surf. B Biointerfaces 2014, 123, 452–460. [Google Scholar] [CrossRef]
- Sadiq, A.A.; Abdul Rassol, A. Formulation and evaluation of silibinin loaded solid lipid nanoparticles for peroral use targeting lower part of gastrointestinal tract. Int. J. Pharm. Pharm. Sci. 2014, 6, 55–67. [Google Scholar]
- Lacatusu, I.; Mitrea, E.; Badea, N.; Stan, R.; Oprea, O.; Meghea, A. Lipid nanoparticles based on omega-3 fatty acids as effective carriers for lutein delivery. Preparation and in vitro characterization studies. J. Funct. Foods 2013, 5, 1260–1269. [Google Scholar] [CrossRef]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lipid nanocarriers for dermal delivery of lutein: Preparation, characterization, stability and performance. Int. J. Pharm. 2011, 414, 267–275. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chiu, H.-C.; Wu, W.-C.; Sahoo, S.L.; Hsu, C.-Y. Novel lutein loaded lipid nanoparticles on porcine corneal distribution. J. Ophthalmol. 2014, 2014, 304694. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Sun, R.; Zhao, G.; Xia, Q. Quercetin loaded nanostructured lipid carrier for food fortification: Preparation, characterization and in vitro study. J. Food Process Eng. 2015, 38, 93–106. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Baskaran, R.; Jang, Y.S.; Oh, S.H.; Yoo, B.K. Quercetin-Loaded Solid Lipid Nanoparticle Dispersion with Improved Physicochemical Properties and Cellular Uptake. AAPS PharmSciTech 2016, 18, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release 2009, 133, 238–244. [Google Scholar] [CrossRef]
- Chen-yu, G.; Chun-fen, Y.; Qi-lu, L.; Qi, T.; Yan-wei, X.; Wei-na, L.; Guang-xi, Z. Development of a quercetin-loaded nanostructured lipid carrier formulation for topical delivery. Int. J. Pharm. 2012, 430, 292–298. [Google Scholar] [CrossRef]
- Liu, C.-H.; Huang, Y.-C.; Jhang, J.-W.; Liu, Y.-H.; Wu, W.-C. Quercetin delivery to porcine cornea and sclera by solid lipid nanoparticles and nanoemulsion. RSC Adv. 2015, 5, 100923–100933. [Google Scholar] [CrossRef]
- Liu, L.; Tang, Y.; Gao, C.; Li, Y.; Chen, S.; Xiong, T.; Li, J.; Du, M.; Gong, Z.; Chen, H. Characterization and biodistribution in vivo of quercetin-loaded cationic nanostructured lipid carriers. Colloids Surf. B Biointerfaces 2014, 115, 125–131. [Google Scholar] [CrossRef]
- Dang, H.; Meng, M.H.W.; Zhao, H.; Iqbal, J.; Dai, R.; Deng, Y.; Lv, F. Luteolin-loaded solid lipid nanoparticles synthesis, characterization, & improvement of bioavailability, pharmacokinetics in vitro and vivo studies. J. Nanopart. Res. 2014, 16, 2347. [Google Scholar]
- Manea, A.-M.; Andronescu, C.; Meghea, A. Green tea extract loaded into solid lipid nanoparticles. UPB Sci. Bull. Ser. B 2014, 76, 125–136. [Google Scholar]
- Ma, Q.H.; Xia, Q.; Lu, Y.Y.; Hao, X.Z.; Gu, N.; Lin, X.F.; Luo, D. Preparation of tea polyphenols-loaded solid lipid nanoparticles based on the phase behaviors of hot microemulsions. Solid State Phenom. 2007, 121–123, 705–708. [Google Scholar] [CrossRef]
- Haddad, F.; Mohammed, N.; Gopalan, R.; Ayoub, Y.A.; Nasim, M.T.; Assi, K. Development and Optimisation of Inhalable EGCG Nano-Liposomes as a Potential Treatment for Pulmonary Arterial Hypertension by Implementation of the Design of Experiments Approach. Pharmaceutics 2023, 15, 539. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.; Neves, A.R.; Sousa, C.T.; Pinheiro, M.; Reis, S. EGCG intestinal absorption and oral bioavailability enhancement using folic acid-functionalized nanostructured lipid carriers. Heliyon 2019, 5, e02020. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos-Villa, M.; Nácher, A.; Hernández, M.; Buso, M.O.V.; Sauri, A.R.; Díez-Sales, O. Mangiferin nanoemulsions in treatment of inflammatory disorders and skin regeneration. Int. J. Pharm. 2019, 564, 299–307. [Google Scholar] [CrossRef]
- Santonocito, D.; Barbagallo, I.; Distefano, A.; Sferrazzo, G.; Vivero-Lopez, M.; Sarpietro, M.G.; Puglia, C. Nanostructured Lipid Carriers Aimed to the Ocular Delivery of Mangiferin: In Vitro Evidence. Pharmaceutics 2023, 15, 951. [Google Scholar] [CrossRef]
- Jaiswal, N.; Akhtar, J.; Khan, M.I.; Ali, A.; Khan, M.F.; Alahmari, A.K. Fabrication and Optimization of Genistein Nanoemulsion. Biochem. Cell. Arch. 2022, 22, 1499–1508. [Google Scholar]
- Gok, B.; Kilinc, Y.B. Chlorogenic acid nanoemulsion for staphylococcus aureus causing skin infection: Synthesis, characterization and evaluation of antibacterial efficacy. Sigma J. Eng. Nat. Sci. 2022, 41, 322–330. [Google Scholar]
- Li, S.; Su, L.; Lv, G.; Luo, W.; Kang, Y. Ultrasound Guided Intra-Articular Injection of Triptolide-loaded Solid Lipid Nanoparticle for Treatment of Antigen-Induced Arthritis in Rabbits. Front. Pharmacol. 2022, 13, 824015. [Google Scholar] [CrossRef]
- Guo, R.-B.; Zhang, X.-Y.; Yan, D.-K.; Yu, Y.-J.; Wang, Y.-J.; Geng, H.-X.; Wu, Y.-N.; Liu, Y.; Kong, L.; Li, X.-T. Folate-modified triptolide liposomes target activated macrophages for safe rheumatoid arthritis therapy. Biomater. Sci. 2022, 10, 499–513. [Google Scholar] [CrossRef]
- Obeid, R.; Kuhlmann, M.; Kirsch, C.-M.; Herrmann, W. Cellular uptake of vitamin B12 in patients with chronic renal failure. Nephron Clin. Pract. 2005, 99, c42–c48. [Google Scholar] [CrossRef]
- Waibel, R.; Treichler, H.; Schaefer, N.G.; van Staveren, D.R.; Mundwiler, S.; Kunze, S.; Küenzi, M.; Alberto, R.; Nüesch, J.; Knuth, A. New derivatives of vitamin B12 show preferential targeting of tumors. Cancer Res. 2008, 68, 2904–2911. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-M.; Cook, N.; Selhub, J.; Manson, J.; Buring, J.; Zhang, S. Plasma folate, vitamin B6, vitamin B12 and risk of breast cancer in women. Cancer Res. 2007, 67, 860. [Google Scholar]
- Genç, L.; Kutlu, H.M.; Güney, G. Vitamin B12-loaded solid lipid nanoparticles as a drug carrier in cancer therapy. Pharm. Dev. Technol. 2015, 20, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yadagiri, G.; Parvez, S.; Singh, O.P.; Verma, A.; Sundar, S.; Mudavath, S.L. Formulation, characterization and in vitro anti-leishmanial evaluation of amphotericin B loaded solid lipid nanoparticles coated with vitamin B12-stearic acid conjugate. Mater. Sci. Eng. C 2020, 117, 111279. [Google Scholar] [CrossRef]
- Çoban, Ö.; Yıldırım, S.; Bakır, T. Alpha-Lipoic Acid and Cyanocobalamin Co-Loaded Nanoemulsions: Development, Characterization, and Evaluation of Stability. J. Pharm. Innov. 2022, 17, 510–520. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Transferrin-functionalized liposomes loaded with vitamin VB12 for Alzheimer's disease therapy. Int. J. Pharm. 2022, 626, 122167. [Google Scholar] [CrossRef]
- Pople, P.V.; Singh, K.K. Development and evaluation of topical formulation containing solid lipid nanoparticles of vitamin A. AAPS PharmSciTech 2006, 7, E63–E69. [Google Scholar] [CrossRef]
- Jenning, V.; Gysler, A.; Schäfer-Korting, M.; Gohla, S.H. Vitamin A loaded solid lipid nanoparticles for topical use: Occlusive properties and drug targeting to the upper skin. Eur. J. Pharm. Biopharm. 2000, 49, 211–218. [Google Scholar] [CrossRef]
- Kim, T.-I.; Kim, T.-G.; Lim, D.-H.; Kim, S.-B.; Park, S.-M.; Hur, T.-Y.; Ki, K.-S.; Kwon, E.-G.; Vijayakumar, M.; Kim, Y.-J. Preparation of Nanoemulsions of Vitamin A and C by Microfluidization: Efficacy on the Expression Pattern of Milk-Specific Proteins in MAC-T Cells. Molecules 2019, 24, 2566. [Google Scholar] [CrossRef]
- Güney, G.; Kutlu, H.M.; Genç, L. Preparation and characterization of ascorbic acid loaded solid lipid nanoparticles and investigation of their apoptotic effects. Colloids Surf. B Biointerfaces 2014, 121, 270–280. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, X.; Han, S.; Zha, X.; Xia, J. Preparation of vitamin C liposomes by rapid expansion of supercritical solution process: Experiments and optimization. J. Drug Deliv. Sci. Technol. 2019, 51, 1–6. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, X.; Zhao, W.; Zeng, C.; Deng, B.; McComb, D.W.; Du, S.; Zhang, C.; Li, W.; Dong, Y. Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat. Nanotechnol. 2020, 15, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Yousry, C.; Saber, M.M.; Abd-Elsalam, W.H. A Cosmeceutical Topical Water-in-Oil Nanoemulsion of Natural Bioactives: Design of Experiment, in vitro Characterization, and in vivo Skin Performance Against UVB Irradiation-Induced Skin Damages. Int. J. Nanomed. 2022, 17, 2995–3012. [Google Scholar] [CrossRef] [PubMed]
- Beers, M.H.; Jones, T.V. The Merck Manual of Health & Aging; Random House Digital, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Nesby-O’Dell, S.; Scanlon, K.S.; Cogswell, M.E.; Gillespie, C.; Hollis, B.W.; Looker, A.C.; Allen, C.; Doughertly, C.; Gunter, E.W.; Bowman, B.A. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Clin. Nutr. 2002, 76, 187–192. [Google Scholar] [CrossRef]
- Ward, L.M.; Gaboury, I.; Ladhani, M.; Zlotkin, S. Vitamin D–deficiency rickets among children in Canada. Can. Med. Assoc. J. 2007, 177, 161–166. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Brody, T. Food and Dietary Supplement Package Labeling—Guidance from FDA's Warning Letters and Title 21 of the Code of Federal Regulations. Compr. Rev. Food Sci. Food Saf. 2016, 15, 92–129. [Google Scholar] [CrossRef]
- Patel, M.R.; Martin-Gonzalez, S.; Fernanda, M. Characterization of ergocalciferol loaded solid lipid nanoparticles. J. Food Sci. 2012, 77, N8–N13. [Google Scholar] [CrossRef]
- Bi, Y.; Xia, H.; Li, L.; Lee, R.J.; Xie, J.; Liu, Z.; Qiu, Z.; Teng, L. Liposomal Vitamin D3 as an Anti-aging Agent for the Skin. Pharmaceutics 2019, 11, 311. [Google Scholar] [CrossRef]
- Maalouf, S.; El-Sabban, M.; Darwiche, N.; Gali-Muhtasib, H. Protective effect of vitamin E on ultraviolet B light-induced damage in keratinocytes. Mol. Carcinog. 2002, 34, 121–130. [Google Scholar] [CrossRef]
- Uemura, M.; Manabe, H.; Yoshida, N.; Fujita, N.; Ochiai, J.; Matsumoto, N.; Takagi, T.; Naito, Y.; Yoshikawa, T. α-Tocopherol prevents apoptosis of vascular endothelial cells via a mechanism exceeding that of mere antioxidation. Eur. J. Pharmacol. 2002, 456, 29–37. [Google Scholar] [CrossRef] [PubMed]
- McVean, M.; Liebler, D.C. Prevention of DNA photodamage by vitamin E compounds and sunscreens: Roles of ultraviolet absorbance and cellular uptake. Mol. Carcinog. 1999, 24, 169–176. [Google Scholar] [CrossRef]
- Dingler, A.; Blum, R.; Niehus, H.; Muller, R.; Gohla, S. Solid lipid nanoparticles (SLNTM/LipopearlsTM) a pharmaceutical and cosmetic carrier for the application of vitamin E in dermal products. J. Microencaps. 1999, 16, 751–767. [Google Scholar]
- Ma, Q.; Wang, Y.; Lin, X.; Luo, D.; Gu, N. Preparation, characterization and photoprotection of tocopherol loaded nanostructured lipid carriers. In Proceedings of the 2007 IEEE/ICME International Conference on Complex Medical Engineering, Beijing, China, 23–27 May 2007; pp. 203–208. [Google Scholar]
- Shahab, M.S.; Rizwanullah, M.; Imam, S.S. Formulation, optimization and evaluation of vitamin E TPGS emulsified dorzolamide solid lipid nanoparticles. J. Drug Deliv. Sci. Technol. 2022, 68, 103062. [Google Scholar] [CrossRef]
- Gaba, B.; Khan, T.; Haider, M.F.; Alam, T.; Baboota, S.; Parvez, S.; Ali, J. Vitamin E Loaded Naringenin Nanoemulsion via Intranasal Delivery for the Management of Oxidative Stress in a 6-OHDA Parkinson’s Disease Model. BioMed Res. Int. 2019, 2019, 2382563. [Google Scholar] [CrossRef]
- Taouzinet, L.; Fatmi, S.; Khellouf, A.; Lahiani-Skiba, M.; Skiba, M.; Iguer-Ouada, M. Drug Release, Stability And Efficiency of Vitamin E Loaded in Liposomes for Bovine Sperm Protection in Cryopreservation Medium. Cryoletters 2022, 43, 50–57. [Google Scholar] [CrossRef]
- Berkner, K.; Runge, K. The physiology of vitamin K nutriture and vitamin K-dependent protein function in atherosclerosis. J. Thromb. Haemost. 2004, 2, 2118–2132. [Google Scholar] [CrossRef]
- IJland, M.M.; Pereira, R.R.; Cornelissen, E.A. Incidence of late vitamin K deficiency bleeding in newborns in The Netherlands in 2005: Evaluation of the current guideline. Eur. J. Pediatr. 2008, 167, 165–169. [Google Scholar] [CrossRef]
- Crowther, M.A.; Douketis, J.D.; Schnurr, T.; Steidl, L.; Mera, V.; Ultori, C.; Venco, A.; Ageno, W. Oral vitamin K lowers the international normalized ratio more rapidly than subcutaneous vitamin K in the treatment of warfarin-associated coagulopathy: A randomized, controlled trial. Ann. Intern. Med. 2002, 137, 251–254. [Google Scholar] [CrossRef]
- Pereira, S.; Shearer, M.; Williams, R.; Mieli-Vergani, G. Intestinal absorption of mixed micellar phylloquinone (vitamin K1) is unreliable in infants with conjugated hyperbilirubinaemia: Implications for oral prophylaxis of vitamin K deficiency bleeding. Arch. Dis. Child.-Fetal Neonatal Ed. 2003, 88, F113–F118. [Google Scholar] [CrossRef]
- Işcan, Y.; Wissing, S.; Müller, R. Solid Lipid Nanoparticles (SLNTM) for topical drug delivery: Incorporation of the lipophilic drugs N, N-diethyl-m-toluamide and vitamin K. Die Pharm. 2005, 60, 905–909. [Google Scholar]
- Liu, C.-H.; Wu, C.-T.; Fang, J.-Y. Characterization and formulation optimization of solid lipid nanoparticles in vitamin K1 delivery. Drug Dev. Ind. Pharm. 2010, 36, 751–761. [Google Scholar] [CrossRef] [PubMed]
- König, A.; Bouzan, C.; Cohen, J.T.; Connor, W.E.; Kris-Etherton, P.M.; Gray, G.M.; Lawrence, R.S.; Savitz, D.A.; Teutsch, S.M. A quantitative analysis of fish consumption and coronary heart disease mortality. Am. J. Prev. Med. 2005, 29, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Awad, T.S.; Helgason, T.; Weiss, J.; Decker, E.A.; McClements, D.J. Effect of omega-3 fatty acids on crystallization, polymorphic transformation and stability of tripalmitin solid lipid nanoparticle suspensions. Cryst. Growth Des. 2009, 9, 3405–3411. [Google Scholar] [CrossRef]
- Muchow, M.; Schmitz, E.; Despatova, N.; Maincent, P.; Müller, R. Omega-3 fatty acids-loaded lipid nanoparticles for patient-convenient oral bioavailability enhancement. Die Pharm. 2009, 64, 499–504. [Google Scholar]
- Salminen, H.; Helgason, T.; Kristinsson, B.; Kristbergsson, K.; Weiss, J. Formation of solid shell nanoparticles with liquid ω-3 fatty acid core. Food Chem. 2013, 141, 2934–2943. [Google Scholar] [CrossRef]
- Blanco-Llamero, C.; Galindo-Camacho, R.M.; Fonseca, J.; Santini, A.; Señoráns, F.J.; Souto, E.B. Development of Lipid Nanoparticles Containing Omega-3-Rich Extract of Microalga Nannochlorpsis gaditana. Foods 2022, 11, 3749. [Google Scholar] [CrossRef]
- Choudhary, P.; Dutta, S.; Moses, J.; Anandharamakrishnan, C. Liposomal encapsulation of omega-3 fatty acid and α-lipoic acid conjugate for cow milk fortification. J. Food Process. Preserv. 2022, 46, e16082. [Google Scholar] [CrossRef]
- Katsouli, M.; Tzia, C. Effect of lipid type, dispersed phase volume fraction and emulsifier on the physicochemical properties of nanoemulsions fortified with conjugated linoleic acid (CLA): Process optimization and stability assessment during storage conditions. J. Mol. Liq. 2019, 292, 111397. [Google Scholar] [CrossRef]
- Vélez, M.A.; Perotti, M.C.; Hynes, E.R.; Gennaro, A.M. Effect of lyophilization on food grade liposomes loaded with conjugated linoleic acid. J. Food Eng. 2019, 240, 199–206. [Google Scholar] [CrossRef]
- Pucek, A.; Niezgoda, N.; Kulbacka, J.; Wawrzeńczyk, C.; Wilk, K.A. Phosphatidylcholine with conjugated linoleic acid in fabrication of novel lipid nanocarriers. Colloids Surf. Physicochem. Eng. Asp. 2017, 532, 377–388. [Google Scholar] [CrossRef]
- Hashemi, F.S.; Farzadnia, F.; Aghajani, A.; Ahmadzadeh NobariAzar, F.; Pezeshki, A. Conjugated linoleic acid loaded nanostructured lipid carrier as a potential antioxidant nanocarrier for food applications. Food Sci. Nutr. 2020, 8, 4185–4195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Cui, X.; Ma, X.; Wang, Z. Preparation, characterization, and evaluation of antioxidant activity and bioavailability of a self-nanoemulsifying drug delivery system (SNEDDS) for buckwheat flavonoids. Acta Biochim. Biophys. Sin. 2020, 52, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Gharekhani, M.; Alirezalu, K.; Roufegarinejad, L.; Azadmard_damirchi, S. Enrichment of buckwheat and lentil based fermented beverages with vitamin E and vitamin C Nano liposomes. J. Food Sci. Technol. 2022, 19, 193–205. [Google Scholar]
- El-Sayed, S.M.; El-Sayed, H.S.; Elgamily, H.M.; Youssef, A.M. Preparation and evaluation of yogurt fortified with probiotics jelly candy enriched with grape seeds extract nanoemulsion. J. Food Process. Preserv. 2022, 46, e16713. [Google Scholar] [CrossRef]
- Montagner, G.E.; Ribeiro, M.F.; Cadoná, F.C.; Franco, C.; Gomes, P. Liposomes loading grape seed extract: A nanotechnological solution to reduce wine-making waste and obtain health-promoting products. Future Foods 2022, 5, 100144. [Google Scholar] [CrossRef]
- Trapani, A.; Esteban, M.Á.; Curci, F.; Manno, D.E.; Serra, A.; Fracchiolla, G.; Espinosa-Ruiz, C.; Castellani, S.; Conese, M. Solid lipid nanoparticles administering antioxidant grape seed-derived polyphenol compounds: A potential application in aquaculture. Molecules 2022, 27, 344. [Google Scholar] [CrossRef]
- Vaishanavi, S.; Preetha, R. Soy protein incorporated nanoemulsion for enhanced stability of probiotic (Lactobacillus delbrueckii subsp. bulgaricus) and its characterization. Mater. Today Proc. 2021, 40, S148–S153. [Google Scholar] [CrossRef]
- Hussein, J.; El-Bana, M.A.; El-Naggar, M.E.; Abdel-Latif, Y.; El-Sayed, S.M.; Medhat, D. Prophylactic effect of probiotics fortified with Aloe vera pulp nanoemulsion against ethanol-induced gastric ulcer. Toxicol. Mech. Methods 2021, 31, 699–710. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Ansari, B.; Gharsallaoui, A. Polyelectrolytes-stabilized liposomes for efficient encapsulation of Lactobacillus rhamnosus and improvement of its survivability under adverse conditions. Food Chem. 2022, 372, 131358. [Google Scholar] [CrossRef]
- Gani, A.; Benjakul, S. Effect of β-glucan stabilized virgin coconut oil nanoemulsion on properties of croaker surimi gel. J. Aquat. Food Prod. Technol. 2019, 28, 194–209. [Google Scholar] [CrossRef]
- Yanagihara, S.; Kasho, N.; Sasaki, K.; Shironaka, N.; Kitayama, Y.; Yuba, E.; Harada, A. pH-Sensitive branched β-glucan-modified liposomes for activation of antigen presenting cells and induction of antitumor immunity. J. Mater. Chem. B 2021, 9, 7713–7724. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Estupiñan, M.V.; Gutierrez-Lopez, G.F.; Cano-Sarmiento, C.; Parra-Escudero, C.O.; Rodriguez-Estrada, M.T.; Garcia-Varela, R.; García, H.S. Stability and characterization of O/W free phytosterols nanoemulsions formulated with an enzymatically modified emulsifier. LWT 2019, 107, 151–157. [Google Scholar] [CrossRef]
- Shrestha, S.C.; Ghebremeskel, K.; White, K.; Minelli, C.; Tewfik, I.; Thapa, P.; Tewfik, S. Formulation and characterization of phytostanol ester solid lipid nanoparticles for the management of hypercholesterolemia: An ex vivo study. Int. J. Nanomed. 2021, 16, 1977–1992. [Google Scholar] [CrossRef]
- Ishaka, A.; Imam, M.U.; Ismail, M. Nanoemulsification of Rice Bran Wax Policosanol Enhances Its Cardio-protective Effects via Modulation of Hepatic Peroxisome Proliferator-activated Receptor gamma in Hyperlipidemic Rats. J. Oleo Sci. 2020, 69, 1287–1295. [Google Scholar] [CrossRef]
- Amante, C.; Esposito, T.; Luccheo, G.; Luccheo, L.; Russo, P.; Del Gaudio, P. Recapsoma®: A Novel Mixture Based on Bergamot, Ipomoea Batatas, Policosanol Extracts and Liposomal Berberine for the Treatment of Hypercholesterolemia. Life 2022, 12, 1162. [Google Scholar] [CrossRef]
- Li, K.; Guo, Z.; Li, H.; Ren, X.; Sun, C.; Feng, Q.; Kou, S.; Li, Q. Nanoemulsion containing Yellow Monascus pigment: Fabrication, characterization, storage stability, and lipase hydrolytic activity in vitro digestion. Colloids Surf. B Biointerfaces 2023, 224, 113199. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, X.; Wang, Y.; Qin, X.; Li, Z. γ-Oryzanol nanoemulsions produced by a low-energy emulsification method: An evaluation of process parameters and physicochemical stability. Food Funct. 2017, 8, 2202–2211. [Google Scholar] [CrossRef]
- Biagi, M.; Minoretti, P.; Bertona, M.; Emanuele, E. Effects of a nutraceutical combination of fermented red rice, liposomal berberine, and curcumin on lipid and inflammatory parameters in patients with mild-to-moderate hypercholesterolemia: An 8-week, open-label, single-arm pilot study. Arch. Med. Sci.-Atheroscler. Dis. 2018, 3, 137–141. [Google Scholar] [CrossRef]
- Raviadaran, R.; Ng, M.H.; Manickam, S.; Chandran, D. Ultrasound-assisted production of palm oil-based isotonic W/O/W multiple nanoemulsion encapsulating both hydrophobic tocotrienols and hydrophilic caffeic acid with enhanced stability using oil-based Sucragel. Ultrason. Sonochem. 2020, 64, 104995. [Google Scholar] [CrossRef]
- Lee, S.-g.; Kalidindi, T.M.; Lou, H.; Gangangari, K.; Punzalan, B.; Bitton, A.; Lee, C.J.; Vargas, H.A.; Park, S.; Bodei, L. γ-Tocotrienol–Loaded Liposomes for Radioprotection from Hematopoietic Side Effects Caused by Radiotherapeutic Drugs. J. Nucl. Med. 2021, 62, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.B.U.; Cetin, M.; Orgul, D.; Taghizadehghalehjoughi, A.; Hacımuftuoglu, A.; Hekimoglu, S. Formulation and in vitro evaluation of topical nanoemulsion and nanoemulsion-based gels containing daidzein. J. Drug Deliv. Sci. Technol. 2019, 52, 189–203. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Wang, J.; Liu, H.; Chen, Y. Preparation and pharmacokinetic study of Daidzein Long-circulating liposomes. Nanoscale Res. Lett. 2019, 14, 321. [Google Scholar] [CrossRef] [PubMed]
- Obinu, A.; Burrai, G.P.; Cavalli, R.; Galleri, G.; Migheli, R.; Antuofermo, E.; Rassu, G.; Gavini, E.; Giunchedi, P. Transmucosal solid lipid nanoparticles to improve genistein absorption via intestinal lymphatic transport. Pharmaceutics 2021, 13, 267. [Google Scholar] [CrossRef]
- Kim, M.-H.; Jeon, Y.-E.; Kang, S.; Lee, J.-Y.; Lee, K.W.; Kim, K.-T.; Kim, D.-D. Lipid nanoparticles for enhancing the physicochemical stability and topical skin delivery of orobol. Pharmaceutics 2020, 12, 845. [Google Scholar] [CrossRef] [PubMed]
- Nemitz, M.C.; von Poser, G.L.; Teixeira, H.F. In vitro skin permeation/retention of daidzein, genistein and glycitein from a soybean isoflavone rich fraction-loaded nanoemulsions and derived hydrogels. J. Drug Deliv. Sci. Technol. 2019, 51, 63–69. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Egea, M.B.; Salas-Mellado, M.d.l.M.; Segura-Campos, M.R. Chia Oil and Mucilage Nanoemulsion: Potential Strategy to Protect a Functional Ingredient. Int. J. Mol. Sci. 2023, 24, 7384. [Google Scholar] [CrossRef]
- Altun, A.; Ugur-Altun, B. Melatonin: Therapeutic and clinical utilization. Int. J. Clin. Pract. 2007, 61, 835–845. [Google Scholar] [CrossRef]
- Wasdell, M.B.; Jan, J.E.; Bomben, M.M.; Freeman, R.D.; Rietveld, W.J.; Tai, J.; Hamilton, D.; Weiss, M.D. A randomized, placebo-controlled trial of controlled release melatonin treatment of delayed sleep phase syndrome and impaired sleep maintenance in children with neurodevelopmental disabilities. J. Pineal Res. 2008, 44, 57–64. [Google Scholar] [CrossRef]
- Jou, M.J.; Peng, T.I.; Yu, P.Z.; Jou, S.B.; Reiter, R.J.; Chen, J.Y.; Wu, H.Y.; Chen, C.C.; Hsu, L.F. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J. Pineal Res. 2007, 43, 389–403. [Google Scholar] [CrossRef]
- Pieper, G.M.; Nilakantan, V.; Nguyen, T.K.; Hilton, G.; Roza, A.M.; Johnson, C.P. Reactive oxygen and reactive nitrogen as signaling molecules for caspase 3 activation in acute cardiac transplant rejection. Antioxid. Redox Signal. 2008, 10, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Tengattini, S.; Reiter, R.J.; Tan, D.X.; Terron, M.P.; Rodella, L.F.; Rezzani, R. Cardiovascular diseases: Protective effects of melatonin. J. Pineal Res. 2008, 44, 16–25. [Google Scholar] [CrossRef]
- Rezzani, R.; Rodella, L.F.; Fraschini, F.; Gasco, M.R.; Demartini, G.; Musicanti, C.; Reiter, R.J. Melatonin delivery in solid lipid nanoparticles: Prevention of cyclosporine A induced cardiac damage. J. Pineal Res. 2009, 46, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Kanikkannan, N.; Andega, S.; Burton, S.; Babu, R.; Singh, M. Formulation and in vitro evaluation of transdermal patches of melatonin. Drug Dev. Ind. Pharm. 2004, 30, 205–212. [Google Scholar] [CrossRef]
- DeMuro, R.L.; Nafziger, A.N.; Blask, D.E.; Menhinick, A.M.; Bertino, J.S. The absolute bioavailability of oral melatonin. J. Clin. Pharmacol. 2000, 40, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Mallo, C.; Zaidan, R.; Galy, G.; Vermeulen, E.; Brun, J.; Chazot, G.; Claustrat, B. Pharmacokinetics of melatonin in man after intravenous infusion and bolus injection. Eur. J. Clin. Pharmacol. 1990, 38, 297–301. [Google Scholar] [CrossRef]
- Priano, L.; Esposti, D.; Esposti, R.; Castagna, G.; De Medici, C.; Fraschini, F.; Gasco, M.R.; Mauro, A. Solid lipid nanoparticles incorporating melatonin as new model for sustained oral and transdermal delivery systems. J. Nanosci. Nanotechnol. 2007, 7, 3596–3601. [Google Scholar] [CrossRef]
- Lundmark, P.O.; Pandi-Perumal, S.; Srinivasan, V.; Cardinali, D.; Rosenstein, R. Melatonin in the eye: Implications for glaucoma. Exp. Eye Res. 2007, 84, 1021–1030. [Google Scholar] [CrossRef]
- Wiechmann, A.F.; Summers, J.A. Circadian rhythms in the eye: The physiological significance of melatonin receptors in ocular tissues. Prog. Retin. Eye Res. 2008, 27, 137–160. [Google Scholar] [CrossRef]
- Jockers, R.; Maurice, P.; Boutin, J.; Delagrange, P. Melatonin receptors, heterodimerization, signal transduction and binding sites: What’s new? Br. J. Pharmacol. 2008, 154, 1182–1195. [Google Scholar] [CrossRef]
- Alcantara-Contreras, S.; Baba, K.; Tosini, G. Removal of melatonin receptor type 1 increases intraocular pressure and retinal ganglion cells death in the mouse. Neurosci. Lett. 2011, 494, 61–64. [Google Scholar] [CrossRef]
- Bucolo, C.; Salomone, S.; Drago, F.; Reibaldi, M.; Longo, A.; Uva, M.G. Pharmacological management of ocular hypertension: Current approaches and future prospective. Curr. Opin. Pharm. 2013, 13, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Bucolo, C.; Drago, F.; Salomone, S.; Pignatello, R. Cationic solid lipid nanoparticles enhance ocular hypotensive effect of melatonin in rabbit. Int. J. Pharm. 2015, 478, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Qiu, X.; Wang, Y.; Song, S.; Cong, Y.; Hao, J. Application and Efficacy of Melatonin Elastic Liposomes in Photoaging Mice. Oxidative Med. Cell. Longev. 2022, 2022, 7135125. [Google Scholar] [CrossRef]
- Ahmad, N.; Khalid, M.S.; Al Ramadhan, A.M.; Alaradi, M.Z.; Al Hammad, M.R.; Ansari, K.; Alqurashi, Y.D.; Khan, M.F.; Albassam, A.A.; Ansari, M.J.; et al. Preparation of melatonin novel-mucoadhesive nanoemulsion used in the treatment of depression. Polym. Bull. 2023, 80, 8093–8132. [Google Scholar] [CrossRef]
- Bentinger, M.; Brismar, K.; Dallner, G. The antioxidant role of coenzyme Q. Mitochondrion 2007, 7, S41–S50. [Google Scholar] [CrossRef]
- Beyer, R.E. Inhibition by coenzyme Q of ethanol-and carbon tetrachloride-stimulated lipid peroxidation in vivo and catalyzed by microsomal and mitochondrial systems. Free Radic. Biol. Med. 1988, 5, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J. Investig. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef]
- Pardeike, J.; Schwabe, K.; Müller, R.H. Influence of nanostructured lipid carriers (NLC) on the physical properties of the Cutanova Nanorepair Q10 cream and the in vivo skin hydration effect. Int. J. Pharm. 2010, 396, 166–173. [Google Scholar] [CrossRef]
- Schwarz, J.C.; Baisaeng, N.; Hoppel, M.; Löw, M.; Keck, C.M.; Valenta, C. Ultra-small NLC for improved dermal delivery of coenyzme Q10. Int. J. Pharm. 2013, 447, 213–217. [Google Scholar] [CrossRef]
- Lohan, S.B.; Bauersachs, S.; Ahlberg, S.; Baisaeng, N.; Keck, C.M.; Müller, R.H.; Witte, E.; Wolk, K.; Hackbarth, S.; Röder, B.; et al. Ultra-small lipid nanoparticles promote the penetration of coenzyme Q10 in skin cells and counteract oxidative stress. Eur. J. Pharm. Biopharm. 2015, 89, 201–207. [Google Scholar] [CrossRef]
- Moschetti, A.; Vine, L.N.; Lethcoe, K.; Dagda, R.K.; Ellison, P.; Ryan, R.O. Assembly and Characterization of Biocompatible Coenzyme Q10-Enriched Lipid Nanoparticles. Lipids 2020, 55, 141–149. [Google Scholar] [CrossRef]
- Sağıroğlu, A.A. Chitosan-coated liposome-containing carbamazepine and coenzyme Q10: Design, optimization and evaluation. J. Liposome Res. 2021, 31, 389–398. [Google Scholar] [CrossRef] [PubMed]
- El-Leithy, E.S.; Makky, A.M.; Khattab, A.M.; Hussein, D.G. Optimization of nutraceutical coenzyme Q10 nanoemulsion with improved skin permeability and anti-wrinkle efficiency. Drug Dev. Ind. Pharm. 2018, 44, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, L.; Jia, A.; Chang, X.; Xue, H. Preparation and characterization of solid lipid nanoparticles loaded traditional Chinese medicine. Int. J. Biol. Macromol. 2006, 38, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Chen, H.; Weng, T.; Yang, Y.; Yang, X. Solid lipid nanoparticle and microemulsion for topical delivery of triptolide. Eur. J. Pharm. Biopharm. 2003, 56, 189–196. [Google Scholar] [CrossRef]
- Mei, Z.; Li, X.; Wu, Q.; Hu, S.; Yang, X. The research on the anti-inflammatory activity and hepatotoxicity of triptolide-loaded solid lipid nanoparticle. Pharmacol. Res. 2005, 51, 345–351. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, T.; Gao, L. Preparation of oridonin-loaded solid lipid nanoparticles and studies on them in vitro and in vivo. Nanotechnology 2006, 17, 5821. [Google Scholar] [CrossRef]
- Shi, F.; Zhao, J.-H.; Liu, Y.; Wang, Z.; Zhang, Y.-T.; Feng, N.-P. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int. J. Nanomed. 2012, 7, 2033–2043. [Google Scholar]
- Wang, S.; Chen, T.; Chen, R.; Hu, Y.; Chen, M.; Wang, Y. Emodin loaded solid lipid nanoparticles: Preparation, characterization and antitumor activity studies. Int. J. Pharm. 2012, 430, 238–246. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, N.; Murariu, A.; Nichita, C.; Bojin, D.; Meghea, A. Antioxidant capacity of lipid nanoparticles loaded with rosemary extract. Mol. Cryst. Liq. Cryst. 2010, 523, 260/[832]–272/[844]. [Google Scholar] [CrossRef]
- Lai, F.; Wissing, S.A.; Müller, R.H.; Fadda, A.M. Artemisia arborescens L essential oil-loaded solid lipid nanoparticles for potential agricultural application: Preparation and characterization. AAPS PharmSciTech 2006, 7, E10–E18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-L.; Yang, C.-R.; Yang, K.-L.; Li, K.-X.; Hu, H.-Y.; Chen, D.-W. Preparation and characterization of nanostructured lipid carriers loaded traditional Chinese medicine, zedoary turmeric oil. Drug Dev. Ind. Pharm. 2010, 36, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.A.; Madureira, A.R.; Sarmento, B.; Gomes, A.M.; Pintado, M.M. Stability of bioactive solid lipid nanoparticles loaded with herbal extracts when exposed to simulated gastrointestinal tract conditions. Food Res. Int. 2015, 78, 131–140. [Google Scholar] [CrossRef]
- Tang, S.; Tan, S.; Yao, L.; Li, F.; Li, L.; Guo, X.; Liu, Y.; Hao, X.; Li, Y.; Ding, X. Risk factors for poor treatment outcomes in patients with MDR-TB and XDR-TB in China: Retrospective multi-center investigation. PLoS ONE 2013, 8, e82943. [Google Scholar] [CrossRef]
- Liu, Z.; Okeke, C.I.; Zhang, L.; Zhao, H.; Li, J.; Aggrey, M.O.; Li, N.; Guo, X.; Pang, X.; Fan, L. Mixed polyethylene glycol-modified breviscapine-loaded solid lipid nanoparticles for improved brain bioavailability: Preparation, characterization, and in vivo cerebral microdialysis evaluation in adult Sprague Dawley rats. AAPS PharmSciTech 2014, 15, 483–496. [Google Scholar] [CrossRef]
- Vijayan, V.; Aafreen, S.; Sakthivel, S.; Reddy, K.R. Formulation and characterization of solid lipid nanoparticles loaded Neem oil for topical treatment of acne. J. Acute Dis. 2013, 2, 282–286. [Google Scholar] [CrossRef]
- Piao, L.; Jin, Y.; Cui, Y.; Yin, S. Preparation of Oenothera biennis Oil Solid Lipid Nanoparticles Based on Microemulsion Technique. J. Chin. Med. Mater. 2015, 38, 1290–1294. [Google Scholar]
- Yang, C.-R.; Zhao, X.-L.; Hu, H.-Y.; Li, K.-X.; Sun, X.; Li, L.; Chen, D.-W. Preparation, optimization and characteristic of huperzine a loaded nanostructured lipid carriers. Chem. Pharm. Bull. 2010, 58, 656–661. [Google Scholar] [CrossRef]
- Kumar, N.; Chaiyasut, C. Hair Growth Promoting Activity of Carthamus Tinctorius Florets Extract-Loaded Nanostructured Lipid Carriers. Int. J. Pharm. Pharm. Sci. 2014, 7, 252–257. [Google Scholar]
- Ratcharin, N.; Wongtrakul, P.; Indranupakorn, R. Preparation of Zingiber officinale Extract Loaded Solid Lipid Nanoparticles. Adv. Mater. Res. 2012, 506, 389–392. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Deepika; Singh, B.K.; Dubey, N.K. Antimicrobial, Aflatoxin B1 Inhibitory and Lipid Oxidation Suppressing Potential of Anethole-Based Chitosan Nanoemulsion as Novel Preservative for Protection of Stored Maize. Food Bioprocess Technol. 2020, 13, 1462–1477. [Google Scholar] [CrossRef]
- Mohammad Gholinezhad, R.; Gholipour Zanjani, N.; Kamran Pirzaman, A.; Zahed, B. Evaluation of drug delivery of allicin liposome based on phosphatidylethanolamine and salep-based hydrogel. J. Appl. Chem. 2023, 18, 107–124. [Google Scholar] [CrossRef]
- Shomorony, A.; Santamaria, C.M.; Zhao, C.; Rwei, A.Y.; Mehta, M.; Zurakowski, D.; Kohane, D.S. Prolonged Duration Local Anesthesia by Combined Delivery of Capsaicin- and Tetrodotoxin-Loaded Liposomes. Anesth. Analg. 2019, 129, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, Q.; Wang, J.; Guan, S.; Cai, D.; Liu, J. Inhibition of growth and metastasis of breast cancer by targeted delivery of 17-hydroxy-jolkinolide B via hyaluronic acid-coated liposomes. Carbohydr. Polym. 2021, 257, 117572. [Google Scholar] [CrossRef]
- Aghazadeh, T.; Bakhtiari, N.; Rad, I.A.; Ramezani, F.J.B.R.A.C. Formulation of kaempferol in nanostructured lipid carriers (NLCs): A delivery platform to sensitization of MDA-MB468 breast cancer cells to paclitaxel. Biointerface Res. Appl. Chem. 2021, 11, 14591–14601. [Google Scholar]
- Gad, H.A.; Abd El-Rahman, F.A.A.; Hamdy, G.M. Chamomile oil loaded solid lipid nanoparticles: A naturally formulated remedy to enhance the wound healing. J. Drug Deliv. Sci. Technol. 2019, 50, 329–338. [Google Scholar] [CrossRef]
- Karamchedu, S.; Tunki, L.; Kulhari, H.; Pooja, D. Morin hydrate loaded solid lipid nanoparticles: Characterization, stability, anticancer activity, and bioavailability. Chem. Phys. Lipids 2020, 233, 104988. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Gambaro, R.; Cisneros, J.S.; Huck-Iriart, C.; Padula, G.; Castro, G.R.; Chain, C.Y.; Islan, G.A. Enhanced anticancer activity of encapsulated geraniol into biocompatible lipid nanoparticles against A549 human lung cancer cells. J. Drug Deliv. Sci. Technol. 2023, 80, 104159. [Google Scholar] [CrossRef]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Boselli, C.; Icaro Cornaglia, A.; Mannucci, B.; Grisoli, P.; Vigani, B.; Ferrari, F. Essential oil-loaded lipid nanoparticles for wound healing. Int. J. Nanomed. 2018, 13, 175–186. [Google Scholar] [CrossRef]
- Cordova, C.A.S.; Locatelli, C.; Winter, E.; Silva, A.H.; Zanetti-Ramos, B.G.; Jasper, R.; Mascarello, A.; Yunes, R.A.; Nunes, R.J.; Creczynski-Pasa, T.B. Solid lipid nanoparticles improve octyl gallate antimetastatic activity and ameliorate its renal and hepatic toxic effects. Anticancer Drugs 2017, 28, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Rodenak-Kladniew, B.; Islan, G.A.; de Bravo, M.G.; Durán, N.; Castro, G.R. Design, characterization and in vitro evaluation of linalool-loaded solid lipid nanoparticles as potent tool in cancer therapy. Colloids Surf. B Biointerfaces 2017, 154, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Wi, T.I.; Won, J.E.; Lee, C.M.; Lee, J.W.; Kang, T.H.; Shin, B.C.; Han, H.D.; Park, Y.M. Efficacy of Combination Therapy with Linalool and Doxorubicin Encapsulated by Liposomes as a Two-in-One Hybrid Carrier System for Epithelial Ovarian Carcinoma. Int. J. Nanomed. 2020, 15, 8427–8436. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, J.H.; Dong, F.R.; Liu, Y. Optimized preparation of ginkgolides A and B long-circulating solid lipid nanoparticles by central composite design and response surface method. Nan Fang Yi Ke Da Xue Xue Bao 2008, 28, 700–703. [Google Scholar]
- Vijayanand, P.; Jyothi, V.; Aditya, N.; Mounika, A. Development and Characterization of Solid Lipid Nanoparticles Containing Herbal Extract: In Vivo Antidepressant Activity. J. Drug Deliv. 2018, 2018, 2908626. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, Y.O.; Kostromicheva, M.M.; Ofitserov, E.N.; Koroleva, M.Y. Nanoemulsions with Amaranth and Sea Buckthorn Oils. Colloid J. 2022, 84, 31–38. [Google Scholar] [CrossRef]
- Riquelme, N.; Sepúlveda, C.; Arancibia, C. Influence of Ternary Emulsifier Mixtures on Oxidative Stability of Nanoemulsions Based on Avocado Oil. Foods 2020, 9, 42. [Google Scholar] [CrossRef]
- Suksuwan, A.; Arour, Z.; Santiworakun, N.; Dahlan, W. Preparation and characterization of black seed oil loaded solid lipid nanoparticles for topical formulations: A preliminary study. AIP Conf. Proc. 2021, 2353, 020003. [Google Scholar] [CrossRef]
- Kildaci, I.; Budama-Kilinc, Y.; Kecel-Gunduz, S.; Altuntas, E. Linseed Oil Nanoemulsions for treatment of Atopic Dermatitis disease: Formulation, characterization, in vitro and in silico evaluations. J. Drug Deliv. Sci. Technol. 2021, 64, 102652. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Chu, L.; Xia, Q. Liposome-chitosan hydrogel bead delivery system for the encapsulation of linseed oil and quercetin: Preparation and in vitro characterization studies. LWT 2020, 117, 108615. [Google Scholar] [CrossRef]
- Nemati, S.; Mohammad Rahimi, H.; Hesari, Z.; Sharifdini, M.; Jalilzadeh Aghdam, N.; Mirjalali, H.; Zali, M.R. Formulation of Neem oil-loaded solid lipid nanoparticles and evaluation of its anti-Toxoplasma activity. BMC Complement. Med. Ther. 2022, 22, 122. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, N.; Devi, L.S.; Kumar, S.; Kamle, M.; Kumar, P.; Mukherjee, A. Neem oil and its nanoemulsion in sustainable food preservation and packaging: Current status and future prospects. J. Agric. Food Res. 2022, 7, 100254. [Google Scholar] [CrossRef]
- Yin, Q.; Song, X.; Yang, P.; Yang, W.; Li, X.; Wang, X.; Wang, S. Incorporation of glycyrrhizic acid and polyene phosphatidylcholine in lipid nanoparticles ameliorates acute liver injury via delivering p65 siRNA. Nanomedicine 2023, 48, 102649. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Martínez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Hanley, A.B.; Whitaker, J.R. The genus allium. Part 2. Crit. Rev. Food Sci. Nutr. 1985, 22, 273–377. [Google Scholar] [CrossRef]
- Ledezma, E.; Apitz-Castro, R. Ajoene the main active compound of garlic (Allium sativum): A new antifungal agent]. Rev. Iberoam. Micol. 2006, 23, 75–80. [Google Scholar] [CrossRef]
- Wencui, Z.; Qi, Z.; Ying, W.; Di, W. Preparation of solid lipid nanoparticles loaded with garlic oil and evaluation of their in vitro and in vivo characteristics. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3742–3750. [Google Scholar]
- Faran, S.A.; Asghar, S.; Khalid, S.H.; Khan, I.U.; Asif, M.; Khalid, I.; Gohar, U.F.; Hussain, T. Hepatoprotective and Renoprotective Properties of Lovastatin-Loaded Ginger and Garlic Oil Nanoemulsomes: Insights into Serum Biological Parameters. Medicina 2019, 55, 579. [Google Scholar] [CrossRef]
- Quach, H.; Le, T.V.; Nguyen, T.T.; Nguyen, P.; Nguyen, C.K.; Dang, L.H. Nano-Lipids Based on Ginger Oil and Lecithin as a Potential Drug Delivery System. Pharmaceutics 2022, 14, 1654. [Google Scholar] [CrossRef]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef]
- Assadpour, E.; Mahdi Jafari, S. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef] [PubMed]
- Muala, W.C.B.; Desobgo, Z.S.C.; Jong, N.E. Optimization of extraction conditions of phenolic compounds from Cymbopogon citratus and evaluation of phenolics and aroma profiles of extract. Heliyon 2021, 7, e06744. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; Youssef, A.M. Potential application of herbs and spices and their effects in functional dairy products. Heliyon 2019, 5, e01989. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Xu, D.; Zhang, J.; Wang, Z.; Wang, S.; Guo, H.; Zhang, K.; Li, Y.; Wang, Y. Rana chensinensis Ovum Oil Based on CO2 Supercritical Fluid Extraction: Response Surface Methodology Optimization and Unsaturated Fatty Acid Ingredient Analysis. Molecules 2020, 25, 4170. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; Balakrishnan, P. Advances in Nutraceuticals and Functional Foods: Concepts and Applications; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Kesanakurti, P.; Ragupathy, S.; Faller, A.C.; Shanmughanandhan, D.; Buongiorno, F.; Della Noce, I.; Lu, Z.; Zhang, Y.; Newmaster, S.G. Development of Hydrolysis Probe-Based qPCR Assays for Panax ginseng and Panax quinquefolius for Detection of Adulteration in Ginseng Herbal Products. Foods 2021, 10, 2705. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, C.; Jin, Z.; Xu, X.; Cai, Y.; Bai, Y. HPTLC-bioautography/SERS screening nifedipine adulteration in food supplement based on Ginkgo biloba. Microchem. J. 2020, 154, 104647. [Google Scholar] [CrossRef]
- Moo-Young, M. Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Ram, M.; Misra, H. Metabolic engineering for the production of plant therapeutic compounds. In Medicinal and Aromatic Plants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 169–207. [Google Scholar]
- Pai, S.; Hebbar, A.; Selvaraj, S. A critical look at challenges and future scopes of bioactive compounds and their incorporations in the food, energy, and pharmaceutical sector. Environ. Sci. Pollut. Res. Int. 2022, 29, 35518–35541. [Google Scholar] [CrossRef]
- Antal, D.S.; Ardelean, F. Chapter 16-Challenges in the nanoscale delivery systems development in the pharmaceutical and nutraceutical markets. In Mitochondrial Dysfunction and Nanotherapeutics; de Oliveira, M.R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 441–458. [Google Scholar]
- Vidal-Casanella, O.; Núñez, O.; Granados, M.; Saurina, J.; Sentellas, S. Analytical Methods for Exploring Nutraceuticals Based on Phenolic Acids and Polyphenols. Appl. Sci. 2021, 11, 8276. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vihol, D.; Mehta, B.; Shah, D.; Patel, M.; Vora, L.K.; Pereira-Silva, M.; Paiva-Santos, A.C. Phytochemical-loaded liposomes for anticancer therapy: An updated review. Nanomedicine 2022, 17, 547–568. [Google Scholar] [CrossRef]
- Choi, M.K.; Song, I.S. Pharmacokinetic Drug-Drug Interactions and Herb-Drug Interactions. Pharmaceutics 2021, 13, 610. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055–6074. [Google Scholar] [CrossRef]
- Milewska, S.; Niemirowicz-Laskowska, K.; Siemiaszko, G.; Nowicki, P.; Wilczewska, A.Z.; Car, H. Current Trends and Challenges in Pharmacoeconomic Aspects of Nanocarriers as Drug Delivery Systems for Cancer Treatment. Int. J. Nanomed. 2021, 16, 6593–6644. [Google Scholar] [CrossRef]
- Agency, E.M. ICH Q8 (R2) Pharmaceutical Development; European Medicines Agency: London, UK, 2017. [Google Scholar]
- Agrahari, V.; Hiremath, P. Challenges associated and approaches for successful translation of nanomedicines into commercial products. Nanomedicine 2017, 12, 819–823. [Google Scholar] [CrossRef] [PubMed]




| Methods | Advantages | Disadvantages |
|---|---|---|
| Hot High-Pressure Homogenization | Large scale production, absence of organic solvent, incorporation of lipophilic and insoluble drugs | Temperature dependent degradation, intricacy of crystallization, unsuitable for heat-sensitive or hydrophilic drugs |
| Cold High-Pressure Homogenization | Suitable method for thermolabile compounds | Large particle size and broader size distribution |
| Ultrasonication | Common laboratory equipment | Wider size distribution, large amount of surfactant required, physical instability |
| Phase Inversion Temperature | Temperature can be reduced by surfactant incorporation, narrow size distribution, high stability | Thermolabile ingredients degradation |
| Precipitation | Rapid and reproducible | Toxicological issues due to solvent residue |
| Coacervation | Simple, avoidance of organic solvent | Applicable only to non-pH dependent drugs and alkaline nature lipids |
| Microemulsion | Scale up method, thermodynamically stable formulation | Multiple steps involved, diluted dispersion acquired |
| Double Emulsion | Applicable to hydrophilic drugs | Large particle size, low entrapment efficiency and drug loading |
| Emulsification | High encapsulation efficiency, low energy input, no thermal stress | Removal of organic solvent |
| Thin Film Hydration | MLVs are prepared, good reproducibility | Low encapsulation efficiency |
| Proliposome | High encapsulation efficiency, fast, simple, large quantity production | Poor reproducibility |
| Ether/Ethanol Injection | Simple, large quantity production, reproducible | Removal of organic solvent, poor encapsulation (in case of ethanol injection method) |
| Solvent Evaporation | Avoidance of heat | Solvent residue may cause toxicological problems, dilute suspension |
| Solvent Injection | Easy and fast, small droplets | Residual solvent, removal of organic solvent |
| Microfluidization | Laboratory and industrial scale method | High amount of solvent residue |
| Self-emulsifying system | Enhanced solubility, prevent the biodegradation of lipophilic drugs | Low drug loading capacity, drug leakage, low stability |
| Phytochemicals | Method of Preparation | Potential Activity against | LNPs | References |
|---|---|---|---|---|
| Anacardic acid | Hot homogenization method | Microbial biofilm | SLN | [47] |
| Apigenin | Microemulsion method | Diabetic neuropathy | SLN | [48] |
| Baicalein | Reverse evaporation method | Acute lung injury | Liposomes | [49] |
| Bakuchiol | Hot homogenization method | Psoriasis | SLN | [50] |
| Betulinic acid | Microemulsion method | Retinal oxidative injury | SLN | [51] |
| Caffeic acid | Lipid film hydration | Alzheimer’s disease | Liposomes | [52] |
| Camphor | Emulsification method | Asthma | NE | [53] |
| Carvacrol | High pressure microfludization | Fungal infection | NE | [54] |
| Chrysin | Homogenization and sonication | Pancreatic cancer | SLN | [55] |
| Cinnamaldehyde | Thin film evaporation | Bacterial infection | Liposomes | [56] |
| Citral | High pressure homogenization | Breast cancer | NLC | [57] |
| Coumarin | Cold homogenization method | Drug-resistant bacterial infection | SLN | [58] |
| Eucalyptol | Thin film hydration | Diabetes-associated vascular endothelial injury | Liposomes | [59] |
| Eugenol | High speed shearing | Wound treatment | NE | [60] |
| Ferulic acid | Solvent evaporation | Colon cancer | SLN | [61] |
| Gallic acid | Double emulsion technique | Oxidation in food fortification | SLN | [62] |
| Hesperetin | Phase inversion temperature method | Glioblastoma | NLC | [63] |
| Honokiol | Low energy shaking method | Glioblastoma | NE | [64] |
| Hypericin | Ultrasonication method | Breast cancer | NE | [65] |
| Hyperoside | Thin film hydration | Hepatocellular carcinoma | Liposomes | [66] |
| Juglone | Thin film hydration | Cystic hydatid disease | Liposomes | [67] |
| Kaempferol | Emulsification method | Glioblastoma | NLC | [68] |
| Lapachol | Hot homogenization method | Breast cancer | NE | [69] |
| Licochalcone | High shear homogenization | Schistosomiasis | SLN | [70] |
| Lignin | High pressure homogenization | Hydrophobicity and oxidative stress | NE | [71] |
| Limonene | Ultrasonication method | Fungal infection (tomato preservation) | NLC | [72] |
| Myricetin | Ultrasonication homogenization method | Colorectal cancer | SLN | [73] |
| Oleanolic acid | Thin film hydration | Hepatocellular carcinoma | Liposomes | [74] |
| Paeoniflorin | Thin film hydration | Rheumatoid arthritis | Liposomes | [75] |
| Parthenolide | Thin film hydration | Cervical cancer | Liposomes | [76] |
| Perillyl alcohol | Hot high pressure homogenization | Brain tumor | NLC | [77] |
| Phloretin | Emulsification method | Inflammation | NE | [78] |
| Pinene | Ethanol injection method | Oxidative stress | Liposomes | [79] |
| Pinocembrin | Thin film hydration | Hyperglycemia | Liposomes | [80] |
| Proanthocyanidins | Homogenization method | Parkinson’s disease | SLN | [81] |
| Procyanidin | Thin film hydration | Oxidative stress | Liposomes | [82] |
| Pterostilbene | Ultrasonication method | Breast cancer | SLN | [83] |
| Pulegone | High shear homogenization | Microbial infection | SLN | [84] |
| Quercetin | High shear homogenization | Diabetes mellitus | NE | [85] |
| Thymol | Emulsification method | Oral infections | NE | [86] |
| Thymoquinone | Ultrasonication method | Breast cancer | NE | [87] |
| Umbelliferone | Thin film hydration | Dalton’s ascites lymphoma | Liposomes | [88] |
| Cancer Type | LNPs | Particle Size (nm) | Preparation Methods | References |
|---|---|---|---|---|
| Lung cancer | SLN | 20–80 | Sol-gel | [144] |
| Liver cancer | NLC | 90 | High-pressure microfluidics | [145] |
| Colon cancer | SLN | <500 | Coacervation | [146] |
| Breast cancer | SLN | 152 | High pressure homogenization | [147] |
| Breast cancer | SLN | 194 | High pressure homogenization | [148] |
| Endometrial cancer | Liposomes | 100–150 | Thin-film hydration | [149] |
| Cervical cancer | Liposome | 350–390 | Thin-film hydration | [150] |
| Hepatocellular cancer | Chitosan coated liposome | 240 | Thin-film hydration | [151] |
| Breast cancer | Surface modified liposomes | 207–297 | Thin-film hydration | [152] |
| Breast cancer | NE | 199 | Nanoemulsification | [153] |
| Lung and liver cancer | SLN | 104.1 | Thin-film ultrasonic hydration method | [154] |
| Breast cancer | SLN | 175–190 | Cold dilution of microemulsion | [155] |
| Liver cancer | NE | 114.7 | Emulsification method | [156] |
| Breast cancer | NE | 40 | Emulsification method | [157] |
| Stomach cancer | NLC | 11–50 | High pressure homogenization | [158] |
| Lung cancer | liposome | 80–100 | Thin-film hydration | [159] |
| Liver cancer | liposome | 90–120 | Thin-film hydration | [160] |
| Phytochemicals | Formulation Technique | Excipients | Indications | Route of Delivery | References |
|---|---|---|---|---|---|
| Sulforaphane, Curcumin | hot melt emulsion | dichloromethane, stearic acid, poloxamer 188 | pancreatic cancer | oral | [215] |
| Sulforaphane, Curcumin | hot melt emulsion | stearic acid, poloxamer 188, chitosan | pancreatic cancer | oral | [216] |
| Thymoquinone | high pressure homogenization | olive oil, soy lecithin, phosphatidylcholine, sorbitol, polysorbate 80, thimerosal | gastroprotective | oral | [217] |
| Rosmarinic acid | hot melt ultrasonication | Witepsol wax, polysorbate 80 | food industry applications | oral | [218] |
| Rosmarinic acid | hot melt ultrasonication | carnauba wax, polysorbate 80 | pharmaceutical/food industry | oral | [219] |
| Rosmarinic acid | hot high pressure homogenization | glycerol monostearate (GMS), polysorbate 80, soy lecithin, hydrogenated soybean phosphatidylcholine (HSPC) | Huntington’s disease | nasal | [220] |
| Astaxanthin | high pressure homogenization | glycerol monostearate, stearic acid, glycerol distearates, polysorbate 20, | food industry/ cosmetics | oral/topical | [221] |
| Astaxanthin | double emulsion solvent displacement | stearic acid, poloxamer 188, lecithin | neurological disorders | nasal | [222] |
| Epigallocatec-hin gallate (EGCG) | multiple emulsion (w/o/w) | soybean phosphatidylcholine, Softisan, poloxamer 188, cetyltrimethylammonium bromide (CTAB), dimethyldioctadecylammonium bromide (DDAB) | glaucoma, age-related macular degeneration | ocular | [223] |
| Silibinin | solvent emulsification and evaporation | tristearin, poloxamer 188, polysorbate 80 | colonic diseases | oral | [224] |
| Lutein | high shear homogenization | glycerol stearate, Tween 80, carnauba wax, fish oil, Tween 80, poloxamer 407 | food industry | oral | [225] |
| Lutein | high pressure homogenization | Plantacare 810, Tween 80, carnauba wax, cetyl palmitate, glyceryl tripalmitate | photoprotection | topical | [226] |
| Lutein | hot sonication | Tween 80 and Span 60, diethylene glycol monoethyl ether, cyclodextrin | corneal diseases | ocular | [227] |
| Quercetin | high pressure homogenization | caprylic/capric triglyceride, glyceryl monostearate, glycerol monolaurate, polyglyceryl-10 laurate, polyglycerol-6 monostearate, sucrose ester | functional food industries | oral | [228] |
| Quercetin | ultrasonication | tripalmitin, lecithin, chitosan, Tween 80 | - | oral | [229] |
| Quercetin | emulsification and solidification | glyceryl monostearate, Tween-80. PEG 400, soy lecithin | colonic diseases | oral | [230] |
| Quercetin | emulsion evaporation–solidification | soy lecithin, glyceryl monostearate, stearic acid, medium-chain triglyceride, D-α-tocopheryl polyethylene glycol succinate (TPGS) | dermal infections | topical | [231] |
| Quercetin | hot sonication | glyceryl palmitostearate, Compritol 888, Tween 40 | H2O2-induced oxidative damages | ocular | [232] |
| Quercetin | emulsification and solidification at low temperature | glycerol monostearate, medium-chain triglycerides, Transcutol, soy lecithin | hepatic, renal, and pulmonary disorders | oral | [233] |
| Luteolin | hot-microemulsion ultrasonic | glycerol monostearate, soybean lecithin, Tween 80 | neurodegenerative, cancerous disorders | oral | [234] |
| Green tea extract | high shear homogenization | glycerol Stearate, lecithin, n-hexadecyl palmitate, Tween 80, Tween 20 | bacterial infection | oral | [235] |
| Green tea | w/o microemulsion | polyoxyethylene stearate, poloxamer 188, glyceryl monostearate | topical application | percutaneous | [236] |
| Green tea extract, EGCG | thin-film rehydration | 1,2-dipalmitoyl-sn-glycerol-3-phosphate-rac-(1-glycerol) (DPPG, sodium salt) and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), cholesterol | pulmonary arterial hypertension | pulmonary | [237] |
| Green tea extract, EGCG | high shear homogenization and ultrasonication | Precirol ATO 5, miglyol 812, Tween 80 | infectious and cancer diseases | oral | [238] |
| Mangiferin | homogenization and ultrasonication | Lipoid S75, polysorbate 80, tocopherol, almond oil | skin regeneration | topical | [239] |
| Mangiferin | homogenization and ultrasonication | Lutrol F68, miglyol 812, Compritol 888 ATO | oxidative stress-related ocular diseases | ocular | [240] |
| Genistein | aqueous titration | olive oil, Sefsol 218, Kolliphor RH40, PEG 200 | cancer treatment | - | [241] |
| Chlorogenic Acid | ultrasonic emulsification | Isodecyl neopentanoate, undecyl alcohol, Caprylic/capric triglyceride, DL-alpha-tocopherol acetate, Transcutol, Labrafil, Pluronic F-68 | skin infection | topical | [242] |
| Triptolide | microemulsion | Precirol ATO 5, Compritol 888 ATO, Geleol, Cremophor RH 40, palmitic acid, stearic acid, sodium cholate | antigen-induced arthritis | intra-articular | [243] |
| Triptolide | thin-film hydration | folate was conjugated with polyethylene glycol-distearoyl phosphatidylethanolamine, cholesterol | rheumatoid arthritis | intravenous injection | [244] |
| Functional Food | LNPs Type | Pharmacological Activity | Limitations to Address | References |
|---|---|---|---|---|
| Conjugated linoleic acids | NE, NLC | anti-diabetogenic, anti-carcinogenic effects, antioxidant, and anti-inflammatory | oxidative instability | [286,287,288,289] |
| Buckwheat | NE | antioxidant, anti-carcinogenic, and anti-inflammatory | low oral bioavailability, poor systemic absorption | [290,291] |
| Grape seed polyphenols | NE, liposome | antimicrobial, anti-inflammatory, anti-carcinogenic, antiviral, and antioxidant | light and temperature instability | [292,293,294] |
| Probiotic lactobacilli | NE, liposome | improve the normal flora, resistance against intrusion of pathogens, including shortening of diarrhea episodes, prevention of antibiotic-related diarrhea, reduction of asthma episodes, and reduction of the incidence of some respiratory infections | processing and storage instability | [295,296,297] |
| β-Glucan | NE, liposome | serum cholesterol reduction, blood sugar regulation, antioxidant activity, and immunomodulatory activity | higher molecular weight, reduced solubility | [298,299] |
| Phytosterols and phytostanols | NE, SLN | hypocholesterolemic activity | a high melting point, waxy consistency, easy crystallization | [300,301] |
| Policosanol | NE, liposome | lipid-lowering/hypocholesterolemic activity, antiplatelet | limited bioavailability (5 to 12%) | [302,303] |
| Yellow Monascus Pigment | NE | anti-mutagenic and anti-carcinogenic properties, antimicrobial activities, potential anti-obesity activities | chemical degradation, instability | [304] |
| γ-Oryzanol | NE | antidiabetic, antioxidant | thermal instability and heat degradation | [305] |
| Monacolin K | liposome | anti-inflammatory, lipid lowering | low intestinal absorption, significant first-pass metabolism | [306] |
| Tocotrienol | NE, liposome | anti-proliferative, antioxidant, anti-inflammatory, anti-carcinogenic | instability and poor bioavailability | [307,308] |
| Soybean daidzein | NE, liposome | antioxidant, anti-inflammatory | intense metabolism and insolubility | [309,310] |
| Soybean genistein | SLN | neuroprotective, anti-carcinogenic | oxidation, heat, and light sensitivity, first-pass metabolism | [311] |
| Soybean orobol | SLN, NLC | anti-aging, antioxidant | light sensitivity, low solubility, first-pass metabolism | [312] |
| Soybean glycitein | NE | anti-aging, antioxidant | bitter taste, low solubility and bioavailability, | [313] |
| Chia mucilage | NE | texturizing agent, film-forming agent, cosmetic ingredient | physical, chemical and biological degradation, instability | [314] |
| Herbs/Spices | Pharmacological Activity | LNP Type | References |
|---|---|---|---|
| Tetrandrine | anti-inflammatory, immunologic, antiallergenic, antiarrhythmic | SLN | [342] |
| Triptolide (TP) | anti-inflammatory, immunosuppressive, antifertility, anti-neoplastic activities | SLN | [343,344] |
| Oridonin | anti-tumour, antibacterial, antioxidative, anti-inflammatory | SLN | [345] |
| Frankincense | antibacterial, antifungal, immuno-stimulating activity, anti-inflammatory, | SLN | [346] |
| Myrrh | antispasmodic, antiseptic, anti-tumour | SLN | [346] |
| Emodin | neuroprotective, anti-carcinogenic | SLN | [347] |
| Rosemary | hepatoprotective, antitumerogenic, antimicrobial, antioxidative | SLN, NLC | [348] |
| Artemisia arborescens L. | antiviral | SLN | [349] |
| Zedoary turmeric oil | antibacterial, anti-tumor, antithrombotic, hepatoprotective | NLC | [350] |
| Sage and Savoury | anti-diarrheal, wound healing activity, anti-inflammatory, antihypertensive | SLN | [351] |
| Breviscapine | antihypertensive, antithrombotic, neuroprotective | SLN | [352,353] |
| Neem oil | anti-inflammatory, antipyretic, antifungal, antibacterial, diuretic | SLN | [354] |
| Oenothera biennis Oil | antihypertensive, anti-inflammatory, sedative, astringent | SLN | [355] |
| Huperzine A | selective inhibitor of acetylcholinesterase | NLC | [356] |
| Carthamus tinctorius | antibacterial, anitoxidant, analgesic, sedative, anticonvulsant, immunosuppressive, anti-inflammatory | NLC | [357] |
| Zingiber Officinale | anti-inflammatory, antibacterial, hepatoprotective, anti-carcinogenic, anti-allergenic | SLN | [358] |
| Anethol | antifungal, antiaflatoxigenic, antioxidant | NE | [359] |
| Allicin | anti-inflammatory, antioxidant, neuroprotective | Liposome | [360] |
| Capsaicin | local anaesthetic | Liposome | [361] |
| 17-hydroxy-jolkinolide B | anti-carcinogenic | Liposome | [362] |
| Kaempferol | anti-carcinogenicr, anti-inflammatory, antioxidant | NLC | [363] |
| Chamomile oil | antibacterial, antifungal, anti-inflammatory, antispasmodic, anti-ulcer, antiviral, sedative effect | SLN | [364] |
| Morin hydrate | anti-carcinogenicr | SLN | [365] |
| Geraniol | anti-carcinogenic | NLC | [366] |
| Eucalyptus oil | antibacterial, antifungal, antiseptic, antihyperglycemic, antioxidant | NLC | [367] |
| Gallic acid | anti-cytotoxic, antiproliferative | SLN | [368] |
| Linalool | antiproliferative agent | SLN, Liposome | [369,370] |
| Ginkgolides A and B | anti-cardiovascular, cerebrovascular | SLN | [371] |
| Hibiscus rosa sinensis | antidepressant, aphrodisiac, abortifacient | SLN | [372] |
| Amaranth oil | anti-tumor, antimicrobial, antifungal | NE | [373] |
| Avocado oil | hypercholesterolemia | NE | [374] |
| Nigella sativa | antioxidative, antimicrobial | SLN | [375] |
| Linseed oil | anti-inflammatory, antitumorigenic | NE, Liposome | [376,377] |
| Neem oil | anti-hemorrhoid, antiprotozoal, antibacterial, antitoxoplasma activity | SLN, NE | [378,379] |
| Glycyrrhizic acid | anti-inflammation, antiviral, detoxification, hepatic protection | modified LNP | [380] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashfaq, R.; Rasul, A.; Asghar, S.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Lipid Nanoparticles: An Effective Tool to Improve the Bioavailability of Nutraceuticals. Int. J. Mol. Sci. 2023, 24, 15764. https://doi.org/10.3390/ijms242115764
Ashfaq R, Rasul A, Asghar S, Kovács A, Berkó S, Budai-Szűcs M. Lipid Nanoparticles: An Effective Tool to Improve the Bioavailability of Nutraceuticals. International Journal of Molecular Sciences. 2023; 24(21):15764. https://doi.org/10.3390/ijms242115764
Chicago/Turabian StyleAshfaq, Rabia, Akhtar Rasul, Sajid Asghar, Anita Kovács, Szilvia Berkó, and Mária Budai-Szűcs. 2023. "Lipid Nanoparticles: An Effective Tool to Improve the Bioavailability of Nutraceuticals" International Journal of Molecular Sciences 24, no. 21: 15764. https://doi.org/10.3390/ijms242115764