Genetic Iron Overload Hampers Development of Cutaneous Leishmaniasis in Mice
Abstract
:1. Introduction
2. Results
2.1. Growth of Leishmania Major Is Transiently Delayed in Hjv−/− Mice during Early Infection
2.2. Cytokine Acute Response to Leishmania Major Is Altered in Hjv−/− Mice
2.3. Genetic Iron Overload Does Not Impact Visceral Disease Progression by Leishmania Infantum despite Induction of the Iron Exporter Ferroportin
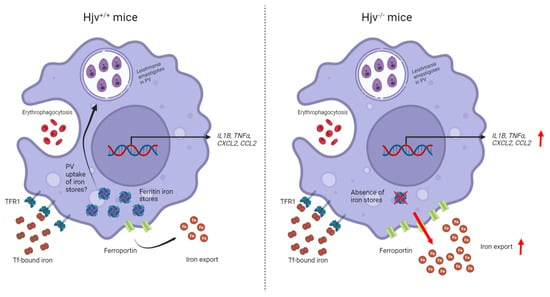
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics
4.2. Parasite Culture
4.3. Footpad Infections
4.4. Limiting Dilution Assay
4.5. Acute Intraperitoneal Infections
4.6. Visceral Leishmaniasis Infection
4.7. Serum Biochemistry
4.8. Quantitative Real-Time PCR (qPCR)
4.9. Multiplex Cytokine/Chemokine Quantification Assay
4.10. Western Blotting
4.11. Tissue Iron Quantification
4.12. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, Y.A.; Andrews, N.W.; Mittra, B. ROS regulate differentiation of visceralizing Leishmania species into the virulent amastigote form. Parasitol. Open 2018, 4, e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Chauhan, N.; Singh, S. Understanding the Cross-Talk of Redox Metabolism and Fe-S Cluster Biogenesis in Leishmania Through Systems Biology Approach. Front. Cell. Infect. Microbiol. 2019, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kevric, I.; Cappel, M.A.; Keeling, J.H. New World and Old World Leishmania Infections. Dermatol. Clin. 2015, 33, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Laskay, T.; van Zandbergen, G.; Solbach, W. Neutrophil granulocytes as host cells and transport vehicles for intracellular pathogens: Apoptosis as infection-promoting factor. Immunobiology 2008, 213, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Van Zandbergen, G.; Klinger, M.; Mueller, A.; Dannenberg, S.; Gebert, A.; Solbach, W.; Laskay, T. Cutting Edge: Neutrophil Granulocyte Serves as a Vector for Leishmania Entry into Macrophages. J. Immunol. 2004, 173, 6521–6525. [Google Scholar] [CrossRef] [Green Version]
- Weiss, G.; Schaible, U.E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef] [Green Version]
- Ganz, T. Iron and infection. Int. J. Hematol. 2018, 107, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Ben-Othman, R.; Flannery, A.R.; Miguel, D.C.; Ward, D.M.; Kaplan, J.; Andrews, N.W. Leishmania-Mediated Inhibition of Iron Export Promotes Parasite Replication in Macrophages. PLoS Pathog. 2014, 10, e1003901. [Google Scholar] [CrossRef]
- Das, N.K.; Sandhya, S.; Vishnu, V.G.; Kumar, R.; Singh, A.K.; Bal, S.K.; Kumari, S.; Mukhopadhyay, C.K. Leishmania donovani inhibits ferroportin translation by modulating FBXL5-IRP2 axis for its growth within host macrophages. Cell. Microbiol. 2018, 20, e12834. [Google Scholar] [CrossRef]
- Chin Shen, C.; Kwang-Poo, C. Heme requirement and acquisition by extracellular and intracellular stages of Leishmania mexicana amazonensis. Mol. Biochem. Parasitol. 1985, 16, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Mittra, B.; Cortez, M.; Haydock, A.; Ramasamy, G.; Myler, P.J.; Andrews, N.W. Iron uptake controls the generation of Leishmania infective forms through regulation of ROS levels. J. Exp. Med. 2013, 210, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Bucheton, B.; Abel, L.; Kheir, M.M.; Mirgani, A.; El-Safi, S.H.; Chevillard, C.; Dessein, A. Genetic control of visceral leishmaniasis in a Sudanese population: Candidate gene testing indicates a linkage to the NRAMP1 region. Genes Immun. 2003, 4, 104–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackwell, J.M.; Fakiola, M.; Ibrahim, M.E.; Jamieson, S.E.; Jeronimo, S.B.; Miller, E.N.; Mishra, A.; Mohamed, H.S.; Peacock, C.S.; Raju, M.; et al. Genetics and visceral leishmaniasis: Of mice and man. Parasite Immunol. 2009, 31, 254–266. [Google Scholar] [CrossRef] [Green Version]
- Castellucci, L.; Jamieson, S.E.; Miller, E.N.; Menezes, E.; Oliveira, J.; Magalhães, A.; Guimarães, L.H.; Lessa, M.; De Jesus, A.R.; Carvalho, E.M.; et al. CXCR1 and SLC11A1polymorphisms affect susceptibility to cutaneous leishmaniasis in Brazil: A case-control and family-based study. BMC Med. Genet. 2010, 11, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, H.W.; Granger, A.M.; Teitelbaum, R.F. Gamma interferon-activated human macrophages and Toxoplasma gondii, Chlamydia psittaci, and Leishmania donovani: Antimicrobial role of limiting intracellular iron. Infect. Immun. 1991, 59, 4684–4686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, N.K.; Biswas, S.; Solanki, S.; Mukhopadhyay, C.K. Leishmania donovani depletes labile iron pool to exploit iron uptake capacity of macrophage for its intracellular growth. Cell. Microbiol. 2009, 11, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Segovia, M.; Navarro, A.; Artero, J.M. The effect of liposome-entrapped desferrioxamine on Leishmania donovani in vitro. Ann. Trop. Med. Parasitol. 1989, 83, 357–360. [Google Scholar] [CrossRef]
- Borges, V.M.; Vannier-Santos, M.A.; De Souza, W. Subverted transferrin trafficking in Leishmania- infected macrophages. Parasitol. Res. 1998, 84, 811–822. [Google Scholar] [CrossRef]
- Bisti, S.; Konidou, G.; Papageorgiou, F.; Milon, G.E.; Boelaert, J.R.; Soteriadou, K. The outcome of Leishmania major experimental infection in BALB / c mice can be modulated by exogenously delivered iron. Eur. J. Immunol. 2000, 30, 3732–3740. [Google Scholar] [CrossRef]
- Vale-Costa, S.; Gomes-Pereira, S.; Teixeira, C.M.; Rosa, G.; Rodrigues, P.N.; Tomás, A.; Appelberg, R.; Gomes, M.S. Iron Overload Favors the Elimination of Leishmania infantum from Mouse Tissues through Interaction with Reactive Oxygen and Nitrogen Species. PLoS Negl. Trop. Dis. 2013, 7, e2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisti, S.; Konidou, G.; Boelaert, J.; Lebastard, M.; Soteriadou, K. The prevention of the growth of Leishmania major progeny in BALB/c iron-loaded mice: A process coupled to increased oxidative burst, the amplitude and duration of which depend on initial parasite developmental stage and dose. Microbes Infect. 2006, 8, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Bisti, S.; Soteriadou, K. Is the reactive oxygen species-dependent-NF-κB activation observed in iron-loaded BALB/c mice a key process preventing growth of Leishmania major progeny and tissue-damage? Microbes Infect. 2006, 8, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; She, H.; Takeuchi, H.; Han, B.; Engelhardt, J.F.; Barton, C.H.; Zandi, E.; Giulivi, C.; Tsukamoto, H. Signaling Role of Intracellular Iron in NF-κB Activation. J. Biol. Chem. 2003, 278, 17646–17654. [Google Scholar] [CrossRef] [Green Version]
- Galaris, D.; Pantopoulos, K. Oxidative Stress and Iron Homeostasis: Mechanistic and Health Aspects. Crit. Rev. Clin. Lab. Sci. 2008, 45, 1–23. [Google Scholar] [CrossRef]
- Artis, D.; Speirs, K.; Joyce, K.; Goldschmidt, M.; Caamaño, J.; Hunter, C.A.; Scott, P. NF-κB1 Is Required for Optimal CD4+ Th1 Cell Development and Resistance to Leishmania major. J. Immunol. 2003, 170, 1995–2003. [Google Scholar] [CrossRef] [Green Version]
- Olynyk, J.K.; Clarke, S.L. Iron overload impairs pro-inflammatory cytokine responses by Kupffer cells. J. Gastroenterol. Hepatol. 2001, 16, 438–444. [Google Scholar] [CrossRef]
- Nairz, M.; Schleicher, U.; Schroll, A.; Sonnweber, T.; Theurl, I.; Ludwiczek, S.; Talasz, H.; Brandacher, G.; Moser, P.L.; Muckenthaler, M.U.; et al. Nitric oxide-mediated regulation of ferroportin-1 controls macrophage iron homeostasis and immune function in Salmonella infection. J. Exp. Med. 2013, 210, 855–873. [Google Scholar] [CrossRef]
- Quinones, M.P.; Estrada, C.A.; Jimenez, F.; Martinez, H.; Willmon, O.; Kuziel, W.A.; Ahuja, S.K.; Ahuja, S.S. CCL2-independent role of CCR2 in immune responses against Leishmania major. Parasite Immunol. 2007, 29, 211–217. [Google Scholar] [CrossRef]
- Weiss, G.; Fuchs, D.; Hausen, A.; Reibnegger, G.; Werner, E.R.; Werner-Felmayer, G.; Wachter, H. Iron modulates interferon-gamma effects in the human myelomonocytic cell line THP-1. Exp. Hematol. 1992, 20, 605–610. [Google Scholar]
- Wang, Z.; Yin, W.; Zhu, L.; Li, J.; Yao, Y.; Chen, F.; Sun, M.; Zhang, J.; Shen, N.; Song, Y.; et al. Iron Drives T Helper Cell Pathogenicity by Promoting RNA-Binding Protein PCBP1-Mediated Proinflammatory Cytokine Production. Immunity 2018, 49, 80–92.e7. [Google Scholar] [CrossRef] [PubMed]
- Pantopoulos, K.; Weiss, G.; Hentze, M.W. Nitric oxide and the post-transcriptional control of cellular iron traffic. Trends Cell Biol. 1994, 4, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Werner-Felmayer, G.; Werner, E.R.; Grünewald, K.; Wachter, H.; Hentze, M.W. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J. Exp. Med. 1994, 180, 969–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef] [PubMed]
- Billesbølle, C.B.; Azumaya, C.M.; Kretsch, R.C.; Powers, A.S.; Gonen, S.; Schneider, S.; Arvedson, T.; Dror, R.O.; Cheng, Y.; Manglik, A. Structure of hepcidin-bound ferroportin reveals iron homeostatic mechanisms. Nature 2020, 586, 807–811. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [Green Version]
- Rishi, G.; Subramaniam, V.N. Signaling pathways regulating hepcidin. Vitam. Horm. 2019, 110, 47–70. [Google Scholar]
- Papanikolaou, G.; Samuels, M.E.; Ludwig, E.H.; Macdonald, M.L.E.; Franchini, P.L.; Dubé, M.-P.; Andres, L.; Macfarlane, J.; Sakellaropoulos, N.; Politou, M.; et al. Mutations in HFE2 cause iron overload in chromosome 1q–linked juvenile hemochromatosis. Nat. Genet. 2004, 36, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.W.; Pinkus, J.L.; Pinkus, G.S.; Fleming, M.D.; Andrews, N.C. A mouse model of juvenile hemochromatosis. J. Clin. Investig. 2005, 115, 2187–2191. [Google Scholar] [CrossRef]
- Pantopoulos, K. Inherited Disorders of Iron Overload. Front. Nutr. 2018, 5, 103. [Google Scholar] [CrossRef] [Green Version]
- Cairo, G.; Recalcati, S.; Montosi, G.; Castrusini, E.; Conte, D.; Pietrangelo, A. Inappropriately High Iron Regulatory Protein Activity in Monocytes of Patients with Genetic Hemochromatosis. Blood 1997, 89, 2546–2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsarou, A.; Gkouvatsos, K.; Fillebeen, C.; Pantopoulos, K. Tissue-Specific Regulation of Ferroportin in Wild-Type and Hjv−/− Mice Following Dietary Iron Manipulations. Hepatol. Commun. 2021, 5, 2139–2150. [Google Scholar] [CrossRef] [PubMed]
- Olakanmi, O.; Schlesinger, L.S.; Britigan, B.E. Hereditary hemochromatosis results in decreased iron acquisition and growth by Mycobacterium tuberculosis within human macrophages. J. Leukoc. Biol. 2007, 81, 195–204. [Google Scholar] [CrossRef]
- Nairz, M.; Theurl, I.; Schroll, A.; Theurl, M.; Fritsche, G.; Lindner, E.; Seifert, M.; Crouch, M.L.; Hantke, K.; Akira, S.; et al. Absence of functional Hfe protects mice from invasive Salmonella enterica serovar Typhimurium infection via induction of lipocalin-2. Blood 2009, 114, 3642–3651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fillebeen, C.; Wilkinson, N.; Charlebois, E.; Katsarou, A.; Wagner, J.; Pantopoulos, K. Hepcidin-mediated hypoferremic response to acute inflammation requires a threshold of Bmp6/Hjv/Smad signaling. Blood 2018, 132, 1829–1841. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [Green Version]
- Nairz, M.; Theurl, I.; Ludwiczek, S.; Theurl, M.; Mair, S.M.; Fritsche, G.; Weiss, G. The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cell. Microbiol. 2007, 9, 2126–2140. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Shen, Y.; Tao, Y.; Wei, J.; Wang, H.; An, P.; Zhang, Z.; Gao, H.; Zhou, T.; Wang, F.; et al. Hemojuvelin regulates the innate immune response to peritoneal bacterial infection in mice. Cell Discov. 2017, 3, 17028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, P.; Natovitz, P.; Coffman, R.L.; Pearce, E.; Sher, A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J. Exp. Med. 1988, 168, 1675–1684. [Google Scholar] [CrossRef]
- Heinzel, F.P.; Sadick, M.D.; Holaday, B.J.; Coffman, R.L.; Locksley, R.M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J. Exp. Med. 1989, 169, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Restrepo, C.M.; Llanes, A.; Herrera, L.; Ellis, E.; Lleonart, R.; Fernández, P.L. Gene expression patterns associated with Leishmania panamensis infection in macrophages from BALB/c and C57BL/6 mice. PLoS Negl. Trop. Dis. 2021, 15, e0009225. [Google Scholar] [CrossRef]
- Kasahara, T.; Hooks, J.J.; Dougherty, S.F.; Oppenheim, J.J. Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J. Immunol. 1983, 130, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Scharton, T.M.; Scott, P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J. Exp. Med. 1993, 178, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, U.; Hesse, A.; Bogdan, C. Minute numbers of contaminant CD8+ T cells or CD11b+CD11c+ NK cells are the source of IFN-γ in IL-12/IL-18-stimulated mouse macrophage populations. Blood 2005, 105, 1319–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titus, R.G.; Sherry, B.; Cerami, A. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J. Exp. Med. 1989, 170, 2097–2104. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro-De-Jesus, A.; Almeida, R.P.; Lessa, H.; Bacellar, O.; Carvalho, E.M. Cytokine profile and pathology in human leishmaniasis. Braz. J. Med. Biol. Res. 1998, 31, 143–148. [Google Scholar] [CrossRef]
- Hatzigeorgiou, D.E.; He, S.; Sobel, J.; Grabstein, K.H.; Hafner, A.; Ho, J.L. IL-6 down-modulates the cytokine-enhanced antileishmanial activity in human macrophages. J. Immunol. 1993, 151, 3682–3692. [Google Scholar] [CrossRef]
- Stäger, S.; Maroof, A.; Zubairi, S.; Sanos, S.L.; Kopf, M.; Kaye, P.M. Distinct roles for IL-6 and IL-12p40 in mediating protection against Leishmania donovani and the expansion of IL-10+ CD4+ T cells. Eur. J. Immunol. 2006, 36, 1764–1771. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Tseng, S.; Horner, R.M.; Tam, C.; Loda, M.; Rollins, B.J. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 2000, 404, 407–411. [Google Scholar] [CrossRef]
- Charlebois, E.; Fillebeen, C.; Katsarou, A.; Rabinovich, A.; Wisniewski, K.; Venkataramani, V.; Michalke, B.; Velentza, A.; Pantopoulos, K. A crosstalk between hepcidin and IRE/IRP pathways controls ferroportin expression and determines serum iron levels in mice. eLife 2022, 11, e81332. [Google Scholar] [CrossRef]
- Malafaia, G.; de Nadai Marcon, L.; de Fátima Pereira, L.; Pedrosa, M.L.; Rezende, S.A. Leishmania chagasi: Effect of the iron deficiency on the infection in BALB/c mice. Exp. Parasitol. 2011, 127, 719–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.A.; Fisher, M.A.; Khakoo, R.A. Association of hemochromatosis with infectious diseases: Expanding spectrum. Int. J. Infect. Dis. 2007, 11, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Huynh, C.; Yuan, X.; Miguel, D.C.; Renberg, R.L.; Protchenko, O.; Philpott, C.C.; Hamza, I.; Andrews, N.W. Heme Uptake by Leishmania amazonensis is Mediated by the Transmembrane Protein LHR1. PLoS Pathog. 2012, 8, e1002795. [Google Scholar] [CrossRef] [Green Version]
- Cabello-Donayre, M.; Orrego, L.M.; Herráez, E.; Vargas, P.; Martínez-García, M.; Campos-Salinas, J.; Pérez-Victoria, I.; Vicente, B.; Marín, J.J.G.; Pérez-Victoria, J.M. Leishmania heme uptake involves LmFLVCRb, a novel porphyrin transporter essential for the parasite. Cell. Mol. Life Sci. 2020, 77, 1827–1845. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Andrews, N.W.; Laranjeira-Silva, M.F. Intracellular iron availability modulates the requirement for Leishmania Iron Regulator 1 (LIR1) during macrophage infections. Int. J. Parasitol. 2019, 49, 423–427. [Google Scholar] [CrossRef]
- Laranjeira-Silva, M.F.; Wang, W.; Samuel, T.K.; Maeda, F.Y.; Michailowsky, V.; Hamza, I.; Liu, Z.; Andrews, N.W. A MFS-like plasma membrane transporter required for Leishmania virulence protects the parasites from iron toxicity. PLOS Pathog. 2018, 14, e1007140. [Google Scholar] [CrossRef] [Green Version]
- Huynh, C.; Sacks, D.L.; Andrews, N.W. A Leishmania amazonensis ZIP family iron transporter is essential for parasite replication within macrophage phagolysosomes. J. Exp. Med. 2006, 203, 2363–2375. [Google Scholar] [CrossRef]
- Gkouvatsos, K.; Fillebeen, C.; Daba, A.; Wagner, J.; Sebastiani, G.; Pantopoulos, K. Iron-dependent regulation of hepcidin in Hjv−/− mice: Evidence that hemojuvelin is dispensable for sensing body iron levels. PLoS ONE 2014, 9, e85530. [Google Scholar] [CrossRef]
- Silva, R.D.; Sacks, D.L. Metacyclogenesis is a major determinant of Leishmania promastigote virulence and attenuation. Infect. Immun. 1987, 55, 2802–2806. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ross, S.L.; Biswas, K.; Rottman, J.; Allen, J.R.; Long, J.; Miranda, L.P.; Winters, A.; Arvedson, T.L. Identification of Antibody and Small Molecule Antagonists of Ferroportin-Hepcidin Interaction. Front. Pharmacol. 2017, 8, 838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daba, A.; Gkouvatsos, K.; Sebastiani, G.; Pantopoulos, K. Differences in activation of mouse hepcidin by dietary iron and parenterally administered iron dextran: Compartmentalization is critical for iron sensing. J. Mol. Med. 2013, 91, 95–102. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charlebois, E.; Li, Y.; Wagner, V.; Pantopoulos, K.; Olivier, M. Genetic Iron Overload Hampers Development of Cutaneous Leishmaniasis in Mice. Int. J. Mol. Sci. 2023, 24, 1669. https://doi.org/10.3390/ijms24021669
Charlebois E, Li Y, Wagner V, Pantopoulos K, Olivier M. Genetic Iron Overload Hampers Development of Cutaneous Leishmaniasis in Mice. International Journal of Molecular Sciences. 2023; 24(2):1669. https://doi.org/10.3390/ijms24021669
Chicago/Turabian StyleCharlebois, Edouard, Yupeng Li, Victoria Wagner, Kostas Pantopoulos, and Martin Olivier. 2023. "Genetic Iron Overload Hampers Development of Cutaneous Leishmaniasis in Mice" International Journal of Molecular Sciences 24, no. 2: 1669. https://doi.org/10.3390/ijms24021669