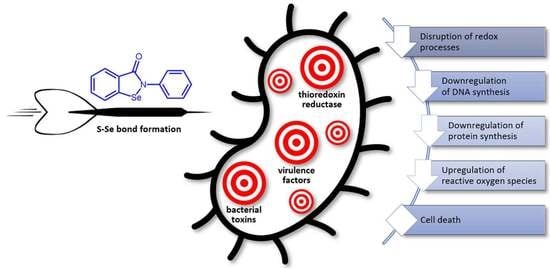
Antibacterial Activity of Ebselen
Abstract
:1. Introduction
2. Antibacterial Activity of Ebselen against Drug-Resistant Bacteria
3. Inhibition of Bacterial Thioredoxin Reductase
4. Synergy of Ebselen with Silver
5. Specific Bacterial Protein Targets for Ebselen
5.1. Clostridium Difficile Toxins
5.2. Urease
5.3. M. tuberculosis Antigen 85 and l,d-Transpeptidase 2
5.4. New Delhi Metallo-β-Lactamase-1
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tong, Z.; Shi, J.; Li, R.; Upton, M.; Wang, Z. Drug repurposing for next-generation combination therapies against multidrug-resistant bacteria. Theranostics 2021, 11, 4910–4928. [Google Scholar] [CrossRef] [PubMed]
- Azad, G.K.; Tomar, R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Cai, Y.; Xiang, C.; Wu, T.; Zhao, Y.; Wang, J.; Wang, H.; Zou, L. Ebselen, a multi-target compound: Its effects on biological processes and diseases. Expert Rev. Mol. Med. 2021, 23, e12. [Google Scholar] [CrossRef]
- Santi, C.; Scimmi, C.; Sancineto, L. Ebselen and analogues: Pharmacological properties and synthetic strategies for their preparation. Molecules 2021, 26, 4230. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Barbosa, N.V.; Rocha, J.B.T. Toxicology and pharmacology of synthetic organoselenium compounds: An update. Arch. Toxicol. 2021, 95, 1179–1226. [Google Scholar] [CrossRef]
- Ruberte, A.C.; Sanmartin, C.; Aydillo, C.; Sharma, A.K.; Plano, D. Development and therapeutic potential of selenazo compounds. J. Med. Chem. 2020, 63, 1473–1489. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Parnham, M.J. Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Radic. Biol. Med. 2020, 156, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, R.; Yokota, T.; Fujimoto, T. Susceptibility of methicillin-resistant Staphylococcus aureus to the selenium-containing compound 2-phenyl-1,2-benzoisoselenazol-3(2H)-one (PZ51). Antimicrob. Agents Chemother. 1989, 33, 1388–1390. [Google Scholar] [CrossRef]
- Younis, W.; Thangamani, S.; Seleem, M.N. Repurposing non-antimicrobial drugs and clinical molecules to treat bacterial infections. Curr. Pharm. Des. 2015, 21, 4106–4111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangamani, S.; Younis, W.; Seleem, M.N. Repurposing clinical molecule ebselen to combat drug resistant pathogens. PLoS ONE 2015, 10, e0133877. [Google Scholar] [CrossRef] [PubMed]
- Thangamani, S.; Younis, W.; Seleem, M.N. Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections. Sci. Rep. 2015, 5, 11596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AbdelKhalek, A.; Abutaleb, N.S.; Mohammad, H.; Seleem, M.N. Repurposing ebselen for decolonization of vancomycin-resistant enterococci (VRE). PLoS ONE 2018, 13, e0199710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, H.; Abutaleb, N.S.; Dieterly, A.M.; Lyle, L.T.; Seleem, M.N. Evaluation of ebselen in resolving a methicillin-resistant Staphylococcus aureus infection of pressure ulcers in obese and diabetic mice. PLoS ONE 2021, 16, e0247508. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.K.; Lee, G.C.; Teng, C.; Frei, C.R. In vitro activity of non-antibiotic drugs against Staphylococcus aureus clinical strains. J. Glob. Antimicrob. Resist. 2021, 27, 167–171. [Google Scholar] [CrossRef]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef]
- Torres, N.S.; Abercrombie, J.J.; Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K.; Leung, K.P. Screening a commercial library of pharmacologically active small molecules against Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2016, 60, 5663–5672. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, S.A.; Priyadarsini, I.K.; Vavilala, S.L. Ebselen’s potential to inhibit planktonic and biofilm growth of Neisseria mucosa. Curr. Chem. Biol. 2022, 16, 61–69. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, X.; Sun, Y.; Li, P.; Xu, M.; Fang, P.; Zhang, S.; Yuan, G.; Deng, X.; Hu, H. Antibiotics-free nanoparticles eradicate Helicobacter pylori biofilms and intracellular bacteria. J. Control. Release 2022, 348, 370–385. [Google Scholar] [CrossRef]
- Lu, J.; Vlamis-Gardikas, A.; Kandasamy, K.; Zhao, R.; Gustafsson, T.N.; Engstrand, L.; Hoffner, S.; Engman, L.; Holmgren, A. Inhibition of bacterial thioredoxin reductase: An antibiotic mechanism targeting bacteria lacking glutathione. FASEB J. 2013, 27, 1394–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Felix, L.; Mylonakis, E.; Fuchs, B.B. Thioredoxin reductase is a valid target for antimicrobial therapeutic development against gram-positive bacteria. Front. Microbiol. 2021, 12, 663481. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zou, L.; Lu, J.; Holmgren, A. Selenocysteine in mammalian thioredoxin reductase and application of ebselen as a therapeutic. Free Radic. Biol. Med. 2018, 127, 238–247. [Google Scholar] [CrossRef]
- Dong, C.; Zhou, J.; Wang, P.; Li, T.; Zhao, Y.; Ren, X.; Lu, J.; Wang, J.; Holmgren, A.; Zou, L. Topical therapeutic efficacy of ebselen against multidrug-resistant Staphylococcus aureus LT-1 targeting thioredoxin reductase. Front. Microbiol. 2020, 10, 3016. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, T.N.; Osman, H.; Werngren, J.; Hoffner, S.; Engman, L.; Holmgren, A. Ebselen and analogs as inhibitors of Bacillus anthracis thioredoxin reductase and bactericidal antibacterials targeting Bacillus species, Staphylococcus aureus and Mycobacterium tuberculosis. Biochim. Biophys. Acta 2016, 1860, 1265–1271. [Google Scholar] [CrossRef]
- Maqbool, I.; Ponniresan, V.K.; Govindasamy, K.; Prasad, N.R. Understanding the survival mechanisms of Deinococcus radiodurans against oxidative stress by targeting thioredoxin reductase redox system. Arch. Microbiol. 2020, 202, 2355–2366. [Google Scholar] [CrossRef]
- Sudharsan, M.; Prasad, N.R.; Kanimozhi, G.; Rishiikeshwer, B.S.; Brindha, G.R.; Chakraborty, A. Redox status and metabolomic profiling of thioredoxin reductase inhibitors and 4 kGy ionizing radiation-exposed Deinococcus radiodurans. Microbiol. Res. 2022, 261, 127070. [Google Scholar] [CrossRef]
- Sudharsan, M.; Prasad, N.R.; Chakraborty, A.; Rajendrasozhan, S. Proteomic profiling of Deinococcus radiodurans with response to thioredoxin reductase inhibitor and ionizing radiation treatment. J. Proteom. 2022, 267, 104697. [Google Scholar] [CrossRef]
- Zou, L.; Lu, J.; Wang, J.; Ren, X.; Zhang, L.; Gao, Y.; Rottenberg, M.E.; Holmgren, A. Synergistic antibacterial effect of silver and ebselen against multidrug-resistant Gram-negative bacterial infections. EMBO Mol. Med. 2017, 9, 1165–1178. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Xie, Z.; Zhou, J.; Lu, Q.; Zhao, Y.; Dong, C.; Zou, L. Depletion of multidrug-resistant uropathogenic Escherichia coli BC1 by ebselen and silver ion. J. Cell Mol. Med. 2020, 24, 13139–13150. [Google Scholar] [CrossRef]
- Dong, C.; Wang, J.; Chen, H.; Wang, P.; Zhou, J.; Zhao, Y.; Zou, L. Synergistic therapeutic efficacy of ebselen and silver ions against multidrug-resistant Acinetobacter baumannii-induced urinary tract infections. Metallomics 2020, 12, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Chen, W.; Zou, L.; Liu, B.; Deng, K.; Guo, D.; Wang, P.; Chen, H.; Wang, H.; Wang, J. The assessment on synergistic activity of ebselen and silver ion against Yersinia pseudotuberculosis. Front. Microbiol. 2022, 13, 963901. [Google Scholar] [CrossRef]
- Wang, X.; Sun, B.; Ye, Z.; Zhang, W.; Xu, W.; Gao, S.; Zhou, N.; Wu, F.; Shen, J. Enzyme-responsive COF-based thiol-targeting nanoinhibitor for curing bacterial infections. ACS Appl. Mater. Interfaces 2022, 14, 38483–38496. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Zhang, H.; Peng, Y.; Deng, F.; Gao, J.; Chai, C.; Tang, S.; Zuo, X.; Lu, J.; et al. Characterization of synergistic antibacterial effect of silver nanoparticles and ebselen. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3338–3349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H. Ebselen. A selenoorganic compound as glutathione peroxidase mimic. Free Radic. Biol. Med. 1993, 14, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Hardej, D.; Santoro, M.; Lau-Cam, C.; Billack, B. Evaluation of the antimicrobial activity of ebselen: Role of the yeast plasma membrane H+-ATPase. J. Biochem. Mol. Toxicol. 2007, 21, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Bender, K.O.; Garland, M.; Ferreyra, J.A.; Hryckowian, A.J.; Child, M.A.; Puri, A.W.; Solow-Cordero, D.E.; Higginbottom, S.K.; Segal, E.; Banaei, N.; et al. A small-molecule antivirulence agent for treating Clostridium difficile infection. Sci. Transl. Med. 2015, 7, 306ra148. [Google Scholar] [CrossRef] [Green Version]
- Guh, A.Y.; Mu, Y.; Winston, L.G.; Johnston, H.; Olson, D.; Farley, M.M.; Wilson, L.E.; Holzbauer, S.M.; Phipps, E.C.; Dumyati, G.K.; et al. Clostridioides difficile Infection Working Group. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N. Engl. J. Med. 2020, 382, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Hryckowian, A.J.; Pruss, K.M.; Sonnenburg, J.L. The emerging metabolic view of Clostridium difficile pathogenesis. Curr. Opin. Microbiol. 2017, 35, 42–47. [Google Scholar] [CrossRef]
- Garland, M.; Hryckowian, A.J.; Tholen, M.; Bender, K.O.; Van Treuren, W.W.; Loscher, S.; Sonnenburg, J.L.; Bogyo, M. The clinical drug ebselen attenuates inflammation and promotes microbiome recovery in mice after antibiotic treatment for CDI. Cell. Rep. Med. 2020, 1, 100005. [Google Scholar] [CrossRef] [PubMed]
- Beilhartz, G.L.; Tam, J.; Zhang, Z.; Melnyk, R.A. Comment on “A small-molecule antivirulence agent for treating Clostridium difficile infection”. Sci. Transl. Med. 2016, 8, 370tc2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marreddy, R.K.R.; Olaitan, A.O.; May, J.N.; Dong, M.; Hurdle, J.G. Ebselen not only inhibits Clostridioides difficile toxins but displays redox-associated cellular killing. Microbiol. Spectr. 2021, 9, e0044821. [Google Scholar] [CrossRef]
- Macegoniuk, K.; Grela, E.; Palus, J.; Rudzińska-Szostak, E.; Grabowiecka, A.; Biernat, M.; Berlicki, Ł. 1,2-Benzisoselenazol-3(2H)-one derivatives as a new class of bacterial urease inhibitors. J. Med. Chem. 2016, 59, 8125–8133. [Google Scholar] [CrossRef] [PubMed]
- Kappaun, K.; Piovesan, A.R.; Carlini, C.R.; Ligabue-Braun, R. Ureases: Historical aspects, catalytic, and non-catalytic properties—A review. J. Adv. Res. 2018, 13, 3–17. [Google Scholar] [CrossRef]
- Mazzei, L.; Musiani, F.; Ciurli, S. The structure-based reaction mechanism of urease, a nickel dependent enzyme: Tale of a long debate. JBIC J. Biol. Inorg. Chem. 2020, 25, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Chiaradia, L.; Lefebvre, C.; Parra, J.; Marcoux, J.; Burlet-Schiltz, O.; Etienne, G.; Tropis, M.; Daffé, M. Dissecting the mycobacterial cell envelope and defining the composition of the native mycomembrane. Sci. Rep. 2017, 7, 12807. [Google Scholar] [CrossRef] [Green Version]
- Favrot, L.; Grzegorzewicz, A.E.; Lajiness, D.H.; Marvin, R.K.; Boucau, J.; Isailovic, D.; Jackson, M.; Ronning, D.R. Mechanism of inhibition of Mycobacterium tuberculosis antigen 85 by ebselen. Nat. Commun. 2013, 4, 2748. [Google Scholar] [CrossRef] [Green Version]
- Amporndanai, K.; Meng, X.; Shang, W.; Jin, Z.; Rogers, M.; Zhao, Y.; Rao, Z.; Liu, Z.-J.; Yang, H.; Zhang, L.; et al. Inhibition mechanism of SARS-CoV-2 main protease by ebselen and its derivatives. Nat. Commun. 2021, 12, 3061. [Google Scholar] [CrossRef]
- Oanca, G.; Asadi, M.; Saha, A.; Ramachandran, B.; Warshel, A. Exploring the catalytic reaction of cysteine proteases. J. Phys. Chem. B 2020, 124, 11349–11356. [Google Scholar] [CrossRef]
- Favrot, L.; Lajiness, D.H.; Ronning, D.R. Inactivation of the Mycobacterium tuberculosis antigen 85 complex by covalent, allosteric inhibitors. J. Biol. Chem. 2014, 289, 25031–25040. [Google Scholar] [CrossRef] [Green Version]
- Goins, C.M.; Dajnowicz, S.; Thanna, S.; Sucheck, S.J.; Parks, J.M.; Ronning, D.R. Exploring covalent allosteric inhibition of antigen 85C from Mycobacterium tuberculosis by ebselen derivatives. ACS Infect. Dis. 2017, 3, 378–387. [Google Scholar] [CrossRef]
- Aliashkevich, A.; Cava, F. LD-transpeptidases: The great unknown among the peptidoglycan cross-linkers. FEBS J. 2022, 289, 4718–4730. [Google Scholar] [CrossRef]
- de Munnik, M.; Lohans, C.T.; Lang, P.A.; Langley, G.W.; Malla, T.R.; Tumber, A.; Schofield, C.J.; Brem, J. Targeting the Mycobacterium tuberculosis transpeptidase LdtMt2 with cysteine-reactive inhibitors including ebselen. Chem. Commun. 2019, 55, 10214–10217. [Google Scholar] [CrossRef] [Green Version]
- Grewal, A.S.; Thapa, K.; Sharma, N.; Singh, S. New Delhi metallo-β-lactamase-1 inhibitors for combating antibiotic drug resistance: Recent developments. Med. Chem. Res. 2020, 29, 1301. [Google Scholar] [CrossRef]
- Linciano, P.; Cendron, L.; Gianquinto, E.; Spyrakis, F.; Tondi, D. Ten years with New Delhi metallo-β-lactamase-1 (NDM-1): From structural insights to inhibitor design. ACS Infect. Dis. 2019, 5, 9. [Google Scholar] [CrossRef]
- Chiou, J.; Wan, S.; Chan, K.-F.; So, P.-K.; He, D.; Chan, E.W.-C.; Chan, T.-H.; Wong, K.-Y.; Tao, J.; Chen, S. Ebselen as a potent covalent inhibitor of New Delhi metallo-β-lactamase (NDM-1). Chem. Commun. 2015, 51, 9543–9546. [Google Scholar] [CrossRef]
- Jin, W.B.; Xu, C.; Cheng, Q.; Qi, X.L.; Gao, W.; Zheng, Z.; Chan, E.W.-C.; Leung, Y.C.; Chan, T.-H.; Wong, K.-Y.; et al. Investigation of synergistic antimicrobial effects of the drug combinations of meropenem and 1,2-benzisoselenazol-3(2H)-one derivatives on carbapenem-resistant Enterobacteriaceae producing NDM-1. Eur. J. Med. Chem. 2018, 155, 285–302. [Google Scholar] [CrossRef]
- Chen, C.; Xiang, Y.; Yang, K.W.; Zhang, Y.; Wang, W.M.; Su, J.P.; Ge, Y.; Liu, Y. A protein structure-guided covalent scaffold selectively targets the B1 and B2 subclass metallo-β-lactamases. Chem. Commun. 2018, 54, 4802–4805. [Google Scholar] [CrossRef]
- Su, J.; Liu, J.; Chen, C.; Zhang, Y.; Yang, K. Ebsulfur as a potent scaffold for inhibition and labelling of New Delhi metallo-β-lactamase-1 in vitro and in vivo. Bioorg. Chem. 2019, 84, 192. [Google Scholar] [CrossRef]
- Jin, W.B.; Xu, C.; Cheung, Q.; Gao, W.; Zeng, P.; Liu, J.; Chan, E.W.-C.; Leung, Y.-C.; Chan, T.-H.; Wong, K.-Y.; et al. Bioisosteric investigation of ebselen: Synthesis and in vitro characterization of 1,2-benzisothiazol-3(2H)-one derivatives as potent New Delhi metallo-β-lactamase inhibitors. Bioorg. Chem. 2020, 100, 103873. [Google Scholar] [CrossRef]
- Pietka-Ottlik, M.; Wójtowicz-Młochowska, H.; Kołodziejczyk, K.; Piasecki, E.; Młochowski, J. New organoselenium compounds active against pathogenic bacteria, fungi and viruses. Chem. Pharm. Bull. 2008, 56, 1423–1427. [Google Scholar] [CrossRef] [Green Version]
- Piętka-Ottlik, M.; Burda-Grabowska, M.; Woźna, M.; Waleńska, J.; Kaleta, R.; Zaczyńska, E.; Piasecki, E.; Giurg, M. Synthesis of new alkylated and methoxylated analogues of ebselen with antiviral and antimicrobial properties. Arkivoc 2017, 2017, 546–556, and the references cited therein. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Yang, K. Ebselen bearing polar functionality: Identification of potent antibacterial agents against multidrug-resistant Gram-negative bacteria. Bioorg. Chem. 2019, 93, 103286. [Google Scholar] [CrossRef]
- Ngo, H.X.; Shrestha, S.K.; Green, K.D.; Garneau-Tsodikova, S. Development of ebsulfur analogues as potent antibacterials against methicillin-resistant Staphylococcus aureus. Bioorg. Med. Chem. 2016, 24, 6298–6306. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Ngo, H.X.; Dennis, E.K.; Thamban, C.N.; DeShong, P.; Garneau-Tsodikova, S.; Lee, V.T. Inhibition of Pseudomonas aeruginosa alginate synthesis by ebselen oxide and its analogues. ACS Infect. Dis. 2021, 7, 1713–1726. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Singh, R.; Dwivedi, S.P.; Gaharwar, U.S.; Meena, R.; Rajamani, P.; Prasad, T. Recent updates on drug resistance in Mycobacterium tuberculosis. J. Appl. Microbiol. 2020, 128, 1547–1567. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, A.; Yoshimoto, T.; Kikuchi, H.; Sano, K.; Saito, I.; Yamaguchi, T.; Yasuhara, H. Ebselen in acute middle cerebral artery occlusion: A placebo-controlled, double-blind clinical trial. Cerebrovasc. Dis. 1999, 9, 112–118. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sano, K.; Takakura, K.; Saito, I.; Shinohara, Y.; Asano, T.; Yasuhara, H. Ebselen in acute ischemic stroke: A placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke 1998, 29, 12–17. [Google Scholar] [CrossRef]
- Kil, J.; Lobarinas, E.; Spankovich, C.; Griffiths, S.K.; Antonelli, P.J.; Lynch, E.D.; Le Prell, C.G. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2017, 390, 969–979. [Google Scholar] [CrossRef]






| Activity of Ebselen | |
|---|---|
| Intracellular mechanisms | Inactivation of bacterial thioredoxin reductase Accumulation of oxidized thioredoxins Upregulation of reactive oxygen species Disruption of cellular redox pathways (downregulation of DNA synthesis and expression of cellular proteins) Inhibition of bacterial proteins, toxins, and virulence factors |
| Extracellular mechanisms | Inhibition of biofilm formation (degradation of the polymeric layer and attenuation of quorum sensing) Downregulation of expression of host proinflammatory cytokines |
| Therapeutic effects | Bactericidal properties (alone and in synergic combination with known antimicrobials) Sensitization to common antimicrobials Recovery of microbiome diversity after antibiotic treatment Host immune response modulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maślanka, M.; Mucha, A. Antibacterial Activity of Ebselen. Int. J. Mol. Sci. 2023, 24, 1610. https://doi.org/10.3390/ijms24021610
Maślanka M, Mucha A. Antibacterial Activity of Ebselen. International Journal of Molecular Sciences. 2023; 24(2):1610. https://doi.org/10.3390/ijms24021610
Chicago/Turabian StyleMaślanka, Marta, and Artur Mucha. 2023. "Antibacterial Activity of Ebselen" International Journal of Molecular Sciences 24, no. 2: 1610. https://doi.org/10.3390/ijms24021610