Biotechnological Approaches to Producing Natural Antioxidants: Anti-Ageing and Skin Longevity Prospects
Abstract
:1. Introduction
2. Skin Ageing: Significance and Causes
2.1. Skin Anatomical Structure and Key Functions
2.2. Skin Ageing
3. Plant-Derived Bioactive Compounds with Anti-Ageing Properties
3.1. The Main Production Routes for Plant-Derived Bioactive Compounds
3.2. Dermo-Protective Action of Plant-Derived Antioxidants
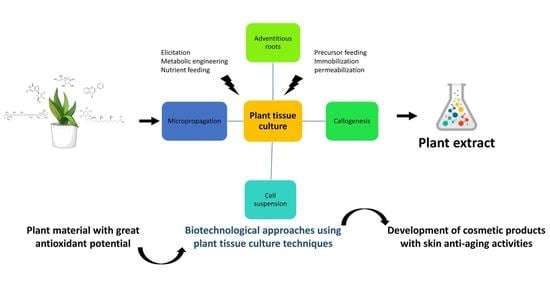
4. Plant Tissue Culture Techniques for the Production of Valuable Antioxidants
4.1. Adventitious Roots and Hairy Roots
4.2. In Vitro Propagation
4.3. Callogenesis and Cell Suspensions
5. Main Biotechnological Approaches to Increasing the Production of Plant-Derived Bioactive Compounds
5.1. Elicitation
5.2. Precursor and Nutrient Feeding
5.3. Metabolic Engineering
5.4. Immobilization
5.5. Permeabilization
6. Production of Antioxidant Substances for Cosmetic Formulations Using Biotechnology
- A patent registered in the United States by Blum et al. in 2012, related to the development of dedifferentiated plant cells from Malus domestica cv Uttwiler Spaetlauber fruits and their use in the formulation of cosmetic preparations to ensure the protection of stem cells against both internal and external stress factors, promotion of stem cell proliferation, and prevention of cell apoptosis (Patent US 8,580,320 B2). From these cell suspensions, different cosmetic preparations have been developed, among which are vanishing creams, liquid balms, intensive hair masks, and eye creams. The efficiency of the developed cosmetic preparations has been tested on stem cells originating from umbilical cords, hair follicles, and fibroblasts.
- Syringa vulgaris plant cells were successfully generated from the in vitro culture of plant tissues under aseptic conditions in growth containers supplemented with specific plant growth regulators by an Italian team (Dal Monte et al., 2006; Patent number: US 7,718,199 B2). An aqueous extraction was performed on callus-derived cell suspensions. HPLC profiling revealed the presence of significant amounts of verbascoside and isoverbascoside. The cell-suspension-derived extracts showed strong antioxidant and scavenging activity against free radicals. Moreover, the developed extracts displayed great anti-hair-loss properties due to their capacity to inhibit 5-alpha reductase and lipoxygenase. The generated extracts also showed strong anti-tyrosinase activity and notable skin-whitening properties.
- Undifferentiated cells of Iris plants (Iris pallida, Iris germanica, and Iris florentina) were generated by Breton and Gueniche in 2001. Galenic preparations were developed from the generated cells. Following the inventors’ claims, the developed preparations included sunscreens, with active ingredients that ensured the protection of the extracellular matrix proteins, such as from UV radiation, through the enzymatic inhibition of MMP proteins (Breton and Gueniche in 2001, Patent number: EP 1 174 120 B1).
- Leontopodium alpinum undifferentiated cells obtained using in vitro cell cultures were used for the formulation of cosmetic preparations by French inventors (Gracioso et al.). The discovery was published as a patent by the inventors in 2016 (Patent deposition in 2015, Patent number: WO 2016/113659 A1). The developed product was proposed as a cosmetic treatment for skin-aged cell homeostasis restoration and increasing cell metabolism and energetic activity.
- Undifferentiated cells of Marrubium vulgare were used as a raw material for the development of cosmetic preparations by Ringenbach et al. for a well-known cosmetic firm. The patent was registered in 2016. For this patent, a cosmetic composition was prepared from plant cells obtained using the in vitro cell culture process. The inventors proposed this cosmetic preparation for topical treatments to improve skin’s general condition, appearance, and appendages, more precisely, for pore tightening and skin imperfections. From the active ingredient discovered, different galenic formulations have been developed, among which are creams, serums, tissue masks, and cleansing lotions (Patent number: WO 2017/163174 A1).
- A cosmetic formulation was developed by an Italian team (Tito et al.) in 2016. The invention covered by this patent focuses on the use of the somatic embryos of three plant species: Lotus japonicus, Citrus limon, and Rosa gardenia. The generated extracts have shown great action against skin-ageing imperfections and contain skin-tissue rejuvenation properties (Patent number: WO 2016/ 173867 A1).
- A cosmetic product with the ability to protect skin from drying and/or prevent UV-radiation damage was developed from a Camellia sinensis var assamica dedifferentiated stem cell culture extraction by Berry et al. The developed product was patented in 2017. The invention’s efficiency was tested in human adult dermal fibroblasts. According to the inventors, the generated tea extracts displayed anti-inflammatory properties, prevented skin cell drying, and protected skin cells from UV radiation (Patent number: WO 2017/178238 A1).
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plant Cell Technology—Your Partner in Plant Tissue Culture. Application of Plant Cell Technology in the Cosmetic Industry. Available online: https://www.plantcelltechnology.com/blog/application-of-plant-cell-technology-in-the-cosmetic-industry/ (accessed on 22 September 2022).
- Precedence Research. Plant Extracts Market Size to Hit Around USD 22.49 Bn by 2030. Available online: https://www.precedenceresearch.com/plant-extracts-market (accessed on 22 September 2022).
- Precedence Research. Plant Extracts Market Size Worth Around USD 22.49 Bn by 2030. Available online: https://www.globenewswire.com/news-release/2022/03/23/2408786/0/en/Plant-Extracts-Market-Size-Worth-Around-USD-22-49-Bn-by-2030.html (accessed on 22 September 2022).
- Trehan, S.; Michniak-Kohn, B.; Beri, K. Plant Stem Cells in Cosmetics: Current Trends and Future Directions. Future Sci. OA 2017, 3, FSO226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiev, V.; Slavov, A.; Vasileva, I.; Pavlov, A. Plant Cell Culture as Emerging Technology for Production of Active Cosmetic Ingredients. Eng. Life Sci. 2018, 18, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In Vitro Plant Tissue Culture: Means for Production of Biological Active Compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Namdeo, A.G.; Ingawale, D.K. Ashwagandha: Advances in Plant Biotechnological Approaches for Propagation and Production of Bioactive Compounds. J. Ethnopharmacol. 2021, 271, 113709. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for Skin Care: A Comprehensive Review of Human Clinical Studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef] [Green Version]
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis; StatPearls Publishing: Treasure Island, FL, USA, 2017. [Google Scholar]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [Green Version]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ricardo-Gonzalez, R.R.; Moro, K. Skin-Resident Innate Lymphoid Cells–Cutaneous Innate Guardi-ans and Regulators. Trends Immunol. 2020, 41, 100–112. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Aryal, E.; Safari, E.; Mojsoska, B.; Jenssen, H.; Prabhala, B.K. Current State of SLC and ABC Trans-porters in the Skin and Their Relation to Sweat Metabolites and Skin Diseases. Proteomes 2021, 9, 23. [Google Scholar] [CrossRef]
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonté, F.; Girard, D.; Archambault, J.-C.; Desmoulière, A. Skin Changes during Ageing. In Biochemistry and Cell Biology of Ageing: Part II Clinical Science; Springer: Berlin/Heidelberg, Germany, 2019; Volume 91, pp. 249–280. [Google Scholar]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamarrón, A.; Lorrio, S.; González, S.; Juarranz, Á. Fernblock Prevents Dermal Cell Damage Induced by Visible and Infrared a Radiation. Int. J. Mol. Sci. 2018, 19, 2250. [Google Scholar] [CrossRef] [Green Version]
- Kammeyer, A.; Luiten, R. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.; Suggs, A.; Baron, E. Ultraviolet Photobiology in Dermatology. In Ultraviolet Light in Human Health, Diseases and Environment; Springer: Berlin/Heidelberg, Germany, 2017; Volume 996, pp. 89–104. [Google Scholar]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Bakrim, W.B.; Nurcahyanti, A.D.R.; Dmirieh, M.; Mahdi, I.; Elgamal, A.M.; El Raey, M.A.; Wink, M.; Sobeh, M. Phytochemical Profiling of the Leaf Extract of Ximenia Americana Var. Caffra and Its Antioxidant, Antibacterial, and Antiaging Activities In Vitro and in Caenorhabditis Elegans: A Cosmeceutical and Dermatological Approach. Oxid. Med. Cell. Longev. 2022, 2022, 3486257. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Wang, M. Bioactive Substances of Plant Origin 30. Handb. Food Chem. 2015, 967, 967–1008. [Google Scholar]
- Abeyrathne, E.D.N.S.; Nam, K.; Huang, X.; Ahn, D.U. Plant-and Animal-Based Antioxidants’ Structure, Efficacy, Mechanisms, and Applications: A Review. Antioxidants 2022, 11, 1025. [Google Scholar] [CrossRef]
- Smetanska, I. Sustainable Production of Polyphenols and Antioxidants by Plant In Vitro Cultures. In Bioprocessing of Plant In Vitro Systems; Springer: Berlin/Heidelberg, Germany, 2018; pp. 225–269. [Google Scholar]
- Namdeo, A. Plant Cell Elicitation for Production of Secondary Metabolites: A Review. Pharmacogn Rev. 2007, 1, 69–79. [Google Scholar]
- Georgiev, M.I.; Weber, J.; Maciuk, A. Bioprocessing of Plant Cell Cultures for Mass Production of Targeted Compounds. Appl. Microbiol. Biotechnol. 2009, 83, 809–823. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.-T.; Sciarappa, W.; Wang, C.Y.; Camp, M.J. Fruit Quality, Antioxidant Capacity, and Flavonoid Content of Organically and Conventionally Grown Blueberries. J. Agric. Food Chem. 2008, 56, 5788–5794. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.C. Production and Engineering of Terpenoids in Plant Cell Culture. Nat. Chem. Biol. 2007, 3, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Coyago-Cruz, E.; Corell, M.; Stinco, C.M.; Hernanz, D.; Moriana, A.; Meléndez-Martínez, A.J. Effect of Regulated Deficit Irrigation on Quality Parameters, Carotenoids and Phenolics of Diverse Tomato Varieties (Solanum Lycopersicum L.). Food Res. Int. 2017, 96, 72–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alquezar, B.; Rodrigo, M.J.; Lado, J.; Zacarías, L. A Comparative Physiological and Transcriptional Study of Carotenoid Biosynthesis in White and Red Grapefruit (Citrus Paradisi Macf.). Tree Genet. Genomes 2013, 9, 1257–1269. [Google Scholar] [CrossRef]
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent Advances in Biorefinery of Astaxanthin from Haematococcus Pluvialis. Bioresour. Technol. 2019, 288, 121606. [Google Scholar] [CrossRef]
- Igreja, W.S.; Maia, F.d.A.; Lopes, A.S.; Chisté, R.C. Biotechnological Production of Carotenoids Using Low Cost-Substrates Is Influenced by Cultivation Parameters: A Review. Int. J. Mol. Sci. 2021, 22, 8819. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Braga, A.; Ferreira, P.; Oliveira, J.; Rocha, I.; Faria, N. Heterologous Production of Resveratrol in Bacterial Hosts: Current Status and Perspectives. World J. Microbiol. Biotechnol. 2018, 34, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Beekwilder, J.; Wolswinkel, R.; Jonker, H.; Hall, R.; de Vos, C.R.; Bovy, A. Production of Resveratrol in Recombinant Microorganisms. Appl. Environ. Microbiol. 2006, 72, 5670–5672. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Schneider, K.; Kristensen, M.; Borodina, I.; Nielsen, J. Engineering Yeast for High-Level Production of Stilbenoid Antioxidants. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, P.; Dudnik, A.; Neves, A.R.; Föster, J. Engineering Lactococcus Lactis for Stilbene Production. In Proceedings of the 28th International Conference on Polyphenols 2016, Vienna, Austria, 11 July 2016; DTU Denmark: Kongens Lyngby, Denmark, 2016. [Google Scholar]
- Kallscheuer, N.; Vogt, M.; Stenzel, A.; Gätgens, J.; Bott, M.; Marienhagen, J. Construction of a Corynebacterium Glutamicum Platform Strain for the Production of Stilbenes and (2S)-Flavanones. Metab. Eng. 2016, 38, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. Resveratrol: A Review of Plant Sources, Synthesis, Stability, Modification and Food Application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, Y.; Li, L.; Linhardt, R.J.; Yan, Y. Regulating Malonyl-CoA Metabolism via Synthetic Antisense RNAs for Enhanced Biosynthesis of Natural Products. Metab. Eng. 2015, 29, 217–226. [Google Scholar] [CrossRef]
- Miras-Moreno, B.; Pedreño, M.Á.; Romero, L.A. Bioactivity and Bioavailability of Phytoene and Strategies to Improve Its Production. Phytochem. Rev. 2019, 18, 359–376. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef] [Green Version]
- Expósito, O.; Bonfill, M.; Moyano, E.; Onrubia, M.; Mirjalili, M.; Cusido, R.; Palazon, J. Biotechnological Production of Taxol and Related Taxoids: Current State and Prospects. Anti-Cancer Agents Med. Chem. Former. Curr. Med. Chem.-Anti-Cancer Agents 2009, 9, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, K.; Kitani, S.; Yoshioka, T.; Morimoto, T.; Fujita, Y.; Yamada, Y. High Density Culture of Coptis Japonica Cells Increases Berberine Production. J. Chem. Technol. Biotechnol. 1989, 46, 61–69. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Srivastava, A.K.; Bhojwani, S.S.; Bisaria, V.S. Production of Podophyllotoxin by Plant Cell Cultures of Podophyllum Hexandrum in Bioreactor. J. Biosci. Bioeng. 2002, 93, 215–220. [Google Scholar] [CrossRef]
- Gao, H.; Xu, J.; Liu, X.; Liu, B.; Deng, X. Light Effect on Carotenoids Production and Expression of Carotenogenesis Genes in Citrus Callus of Four Genotypes. Acta Physiol. Plant. 2011, 33, 2485–2492. [Google Scholar] [CrossRef]
- Buranasudja, V.; Rani, D.; Malla, A.; Kobtrakul, K.; Vimolmangkang, S. Insights into Antioxidant Activities and Anti-Skin-Aging Potential of Callus Extract from Centella Asiatica (L.). Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Kikowska, M.A.; Chmielewska, M.; Włodarczyk, A.; Studzińska-Sroka, E.; Żuchowski, J.; Stochmal, A.; Kotwicka, M.; Thiem, B. Effect of Pentacyclic Triterpenoids-Rich Callus Extract of Chaenomeles Japonica (Thunb.) Lindl. Ex Spach on Viability, Morphology, and Proliferation of Normal Human Skin Fibroblasts. Molecules 2018, 23, 3009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hseu, Y.-C.; Korivi, M.; Lin, F.-Y.; Li, M.-L.; Lin, R.-W.; Wu, J.-J.; Yang, H.-L. Trans-Cinnamic Acid Attenuates UVA-Induced Photoaging through Inhibition of AP-1 Activation and Induction of Nrf2-Mediated Antioxidant Genes in Human Skin Fibroblasts. J. Dermatol. Sci. 2018, 90, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, D.; Panthi, V.K.; Pangeni, R.; Kim, H.J.; Park, J.W. Preparation, Characterization, and Biological Activities of Topical Anti-Aging Ingredients in a Citrus Junos Callus Extract. Molecules 2017, 22, 2198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.; Lee, H.; Tran, Q.; Bayarmunkh, C.; Boldbaatar, D.; Kwon, S.H.; Park, J.; Park, J. Beneficial Effects of Diplectria Barbata (Wall. Ex CB Clarke) Franken et Roos Extract on Aging and Antioxidants in Vitro and in Vivo. Toxicol. Res. 2021, 37, 71–83. [Google Scholar] [CrossRef]
- Menbari, A.; Bahramnejad, B.; Abuzaripoor, M.; Shahmansouri, E.; Zarei, M.A. Establishment of Callus and Cell Suspension Cultures of Granny Smith Apple Fruit and Antityrosinase Activity of Their Extracts. Sci. Hortic. 2021, 286, 110222. [Google Scholar] [CrossRef]
- Machała, P.; Liudvytska, O.; Kicel, A.; Dziedzic, A.; Olszewska, M.A.; Żbikowska, H.M. Valorization of the Photo-Protective Potential of the Phytochemically Standardized Olive (Olea Europaea L.) Leaf Extract in UVA-Irradiated Human Skin Fibroblasts. Molecules 2022, 27, 5144. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Tran, Q.; Cho, H.; Kim, M.; Kim, C.; Kwon, S.H.; Park, S.; Park, J.; Park, J. A New Role for the Ginsenoside RG3 in Antiaging via Mitochondria Function in Ultraviolet-Irradiated Human Dermal Fibroblasts. J. Ginseng Res. 2019, 43, 431–441. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Kwon, S.H.; Park, J.; Park, J. Anti-Aging Effects of Piper Cambodianum P. Fourn. Extract on Normal Human Dermal Fibroblast Cells and a Wound-Healing Model in Mice. Clin. Interv. Aging 2016, 11, 1017. [Google Scholar]
- Rani, D.; Buranasudja, V.; Kobtrakul, K.; De-Eknamkul, W.; Vimolmangkang, S. Elicitation of Pueraria Candollei Var. Mirifica Suspension Cells Promises Antioxidant Potential, Implying Antiaging Activity. Plant Cell Tissue Organ Cult. PCTOC 2021, 145, 29–41. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.W. Anti-Aging Activities of Pyrus Pyrifolia Var Culta Plant Callus Extract. Trop. J. Pharm. Res. 2017, 16, 1579–1588. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-R.; Kim, S.; Jie, E.Y.; Kim, S.J.; Ahn, W.S.; Jeong, S.-I.; Yu, K.-Y.; Kim, S.W.; Kim, S.-Y. Effects of Tiarella Polyphylla D. Don Callus Extract on Photoaging in Human Foreskin Fibroblasts Hs68 Cells. Nat. Prod. Commun. 2021, 16, 1934578X211016970. [Google Scholar] [CrossRef]
- Chalageri, G.; Dhananjaya, S.; Raghavendra, P.; Kumar, L.S.; Babu, U.; Varma, S.R. Substituting Plant Vegetative Parts with Callus Cell Extracts: Case Study with Woodfordia Fruticosa Kurz.–A Potent Ingredient in Skin Care Formulations. S. Afr. J. Bot. 2019, 123, 351–360. [Google Scholar] [CrossRef]
- Zhao, P.; Alam, M.B.; Lee, S.-H. Protection of UVB-Induced Photoaging by Fuzhuan-Brick Tea Aqueous Extract via MAPKs/Nrf2-Mediated down-Regulation of MMP-1. Nutrients 2018, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.-C.; Tsai, Y.-C.; Huang, P.-J.; Ou, T.-T.; Korivi, M.; Hsu, L.-S.; Chang, S.-H.; Wu, C.-R.; Yang, H.-L. The Dermato-Protective Effects of Lucidone from Lindera Erythrocarpa through the Induction of Nrf2-Mediated Antioxidant Genes in UVA-Irradiated Human Skin Keratinocytes. J. Funct. Foods 2015, 12, 303–318. [Google Scholar] [CrossRef]
- Cho, W.K.; Kim, H.-I.; Kim, S.-Y.; Seo, H.H.; Song, J.; Kim, J.; Shin, D.S.; Jo, Y.; Choi, H.; Lee, J.H. Anti-Aging Effects of Leontopodium Alpinum (Edelweiss) Callus Culture Extract through Transcriptome Profiling. Genes 2020, 11, 230. [Google Scholar] [CrossRef] [Green Version]
- Vichit, W.; Saewan, N. Anti-Oxidant and Anti-Aging Activities of Callus Culture from Three Rice Varieties. Cosmetics 2022, 9, 79. [Google Scholar] [CrossRef]
- Kunchana, K.; Jarisarapurin, W.; Chularojmontri, L.; Wattanapitayakul, S.K. Potential Use of Amla (Phyllanthus Emblica L.) Fruit Extract to Protect Skin Keratinocytes from Inflammation and Apoptosis after UVB Irradiation. Antioxidants 2021, 10, 703. [Google Scholar] [CrossRef]
- Farràs, A.; Mitjans, M.; Maggi, F.; Caprioli, G.; Vinardell, M.P.; López, V. Polypodium Vulgare L. (Polypodiaceae) as a Source of Bioactive Compounds: Polyphenolic Profile, Cytotoxicity and Cytoprotective Properties in Different Cell Lines. Front. Pharmacol. 2021, 12, 727528. [Google Scholar] [CrossRef]
- Park, D.E.; Adhikari, D.; Pangeni, R.; Panthi, V.K.; Kim, H.J.; Park, J.W. Preparation and Characterization of Callus Extract from Pyrus Pyrifolia and Investigation of Its Effects on Skin Regeneration. Cosmetics 2018, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Sobeh, M.; Petruk, G.; Osman, S.; El Raey, M.A.; Imbimbo, P.; Monti, D.M.; Wink, M. Isolation of Myricitrin and 3,5-Di-O-Methyl Gossypetin from Syzygium Samarangense and Evaluation of Their Involvement in Protecting Keratinocytes against Oxidative Stress via Activation of the Nrf-2 Pathway. Molecules 2019, 24, 1839. [Google Scholar] [CrossRef] [Green Version]
- Zahid, N.A.; Jaafar, H.Z.; Hakiman, M. Micropropagation of Ginger (Zingiber Officinale Roscoe)‘Bentong’and Evaluation of Its Secondary Metabolites and Antioxidant Activities Compared with the Conventionally Propagated Plant. Plants 2021, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Hyun, T.K. Ectopic Expression of Production of Anthocyanin Pigment 1 (PAP1) Improves the Antioxidant and Anti-Melanogenic Properties of Ginseng (Panax Ginseng CA Meyer) Hairy Roots. Antioxidants 2020, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.M.; Zappelli, C.; Apone, F.; Barbulova, A.; Tito, A.; Leone, A.; Oliviero, T.; Ferracane, R.; Fogliano, V.; Colucci, G. Brassica Rapa Hairy Root Extracts Promote Skin Depigmentation by Modulating Melanin Production and Distribution. J. Cosmet. Dermatol. 2018, 17, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Petruk, G.; Illiano, A.; Del Giudice, R.; Raiola, A.; Amoresano, A.; Rigano, M.M.; Piccoli, R.; Monti, D.M. Malvidin and Cyanidin Derivatives from Açai Fruit (Euterpe Oleracea Mart.) Counteract UV-A-Induced Oxidative Stress in Immortalized Fibroblasts. J. Photochem. Photobiol. B 2017, 172, 42–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apone, F.; Tito, A.; Carola, A.; Arciello, S.; Tortora, A.; Filippini, L.; Monoli, I.; Cucchiara, M.; Gibertoni, S.; Chrispeels, M.J. A Mixture of Peptides and Sugars Derived from Plant Cell Walls Increases Plant Defense Responses to Stress and Attenuates Ageing-Associated Molecular Changes in Cultured Skin Cells. J. Biotechnol. 2010, 145, 367–376. [Google Scholar] [CrossRef]
- Sun, Z.; Park, S.Y.; Hwang, E.; Zhang, M.; Seo, S.A.; Lin, P.; Yi, T. Thymus Vulgaris Alleviates UVB Irradiation Induced Skin Damage via Inhibition of MAPK/AP-1 and Activation of Nrf2-ARE Antioxidant System. J. Cell. Mol. Med. 2017, 21, 336–348. [Google Scholar] [CrossRef]
- Tito, A.; Carola, A.; Bimonte, M.; Barbulova, A.; Arciello, S.; de Laurentiis, F.; Monoli, I.; Hill, J.; Gibertoni, S.; Colucci, G. A Tomato Stem Cell Extract, Containing Antioxidant Compounds and Metal Chelating Factors, Protects Skin Cells from Heavy Metal-induced Damages. Int. J. Cosmet. Sci. 2011, 33, 543–552. [Google Scholar] [CrossRef]
- Jiao, J.; Gai, Q.-Y.; Wang, X.; Qin, Q.-P.; Wang, Z.-Y.; Liu, J.; Fu, Y.-J. Chitosan Elicitation of Isatis Tinctoria L. Hairy Root Cultures for Enhancing Flavonoid Productivity and Gene Expression and Related Antioxidant Activity. Ind. Crops Prod. 2018, 124, 28–35. [Google Scholar] [CrossRef]
- Isah, T.; Umar, S.; Mujib, A.; Sharma, M.P.; Rajasekharan, P.; Zafar, N.; Frukh, A. Secondary Metabolism of Pharmaceuticals in the Plant in Vitro Cultures: Strategies, Approaches, and Limitations to Achieving Higher Yield. Plant Cell Tissue Organ Cult. PCTOC 2018, 132, 239–265. [Google Scholar] [CrossRef]
- Lee, K.-J.; Park, Y.; Kim, J.-Y.; Jeong, T.-K.; Yun, K.-S.; Paek, K.-Y.; Park, S.-Y. Production of Biomass and Bioactive Compounds from Adventitious Root Cultures of Polygonum Multiflorum Using Air-Lift Bioreactors. J. Plant Biotechnol. 2015, 42, 34–42. [Google Scholar] [CrossRef]
- Sharma, P.; Padh, H.; Shrivastava, N. Hairy Root Cultures: A Suitable Biological System for Studying Secondary Metabolic Pathways in Plants. Eng. Life Sci. 2013, 13, 62–75. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Królicka, A.; Wysokińska, H. Establishment of Salvia Officinalis L. Hairy Root Cultures for the Production of Rosmarinic Acid. Z. Für Naturforschung C 2006, 61, 351–356. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Grzegorczyk-Karolak, I.; Frydrych, B.; Królicka, A.; Wysokińska, H. Hairy Roots of Dracocephalum Moldavica: Rosmarinic Acid Content and Antioxidant Potential. Acta Physiol. Plant. 2013, 35, 2095–2103. [Google Scholar] [CrossRef]
- Srivastava, S.; Conlan, X.A.; Adholeya, A.; Cahill, D.M. Elite Hairy Roots of Ocimum Basilicum as a New Source of Rosmarinic Acid and Antioxidants. Plant Cell Tissue Organ Cult. PCTOC 2016, 126, 19–32. [Google Scholar] [CrossRef]
- Shekarchi, M.; Hajimehdipoor, H.; Saeidnia, S.; Gohari, A.R.; Hamedani, M.P. Comparative Study of Rosmarinic Acid Content in Some Plants of Labiatae Family. Pharmacogn. Mag. 2012, 8, 37. [Google Scholar]
- Apone, F.; Tito, A.; Arciello, S.; Carotenuto, G.; Colucci, M.G. Plant Tissue Cultures as Sources of Ingredients for Skin Care Applications. Annu. Plant Rev. Online 2018, 3, 135–150. [Google Scholar]
- Ono, N.N.; Tian, L. The Multiplicity of Hairy Root Cultures: Prolific Possibilities. Plant Sci. 2011, 180, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Bang, S.; Ahn, M.-A.; Lee, K.; Kim, K.; Hyun, T.K. Overproduction of Anthocyanin in Ginseng Hairy Roots Enhances Their Antioxidant, Antimicrobial, and Anti-Elastase Activities. J. Plant Biotechnol. 2021, 48, 100–105. [Google Scholar] [CrossRef]
- Bouzroud, S.; El Maaiden, E.; Sobeh, M.; Devkota, K.P.; Boukcim, H.; Kouisni, L.; El Kharrassi, Y. Micropropagation of Opuntia and Other Cacti Species Through Axillary Shoot Proliferation: A Comprehensive Review. Front. Plant Sci. 2022, 13, 926653. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Romano, A. In Vitro Culture of Lavenders (Lavandula Spp.) and the Production of Secondary Metabolites. Biotechnol. Adv. 2013, 31, 166–174. [Google Scholar] [CrossRef]
- Goyali, J.; Igamberdiev, A.; Debnath, S. Micropropagation Affects Not Only the Fruit Morphology of Lowbush Blueberry (Vaccinium Angustifolium Ait.) but Also Its Medicinal Properties. In Proceedings of the International Symposium on Medicinal Plants and Natural Products, Montreal, QC, Canada, 17–19 June 2013; pp. 137–142. [Google Scholar]
- Dakah, A.; Zaid, S.; Suleiman, M.; Abbas, S.; Wink, M. In Vitro Propagation of the Medicinal Plant Ziziphora Tenuior L. and Evaluation of Its Antioxidant Activity. Saudi J. Biol. Sci. 2014, 21, 317–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sooriamuthu, S.; Varghese, R.J.; Bayyapureddy, A.; John, S.S.T.; Narayanan, R. Light-Induced Production of Antidepressant Compounds in Etiolated Shoot Cultures of Hypericum Hookerianum Wight & Arn.(Hypericaceae). Plant Cell Tissue Organ Cult. PCTOC 2013, 115, 169–178. [Google Scholar]
- Grzegorczyk, I.; Matkowski, A.; Wysokińska, H. Antioxidant Activity of Extracts from in Vitro Cultures of Salvia Officinalis L. Food Chem. 2007, 104, 536–541. [Google Scholar] [CrossRef]
- Al Khateeb, W.; Hussein, E.; Qouta, L.; Alu’datt, M.; Al-Shara, B.; Abu-Zaiton, A. In Vitro Propagation and Characterization of Phenolic Content along with Antioxidant and Antimicrobial Activities of Cichorium Pumilum Jacq. Plant Cell Tissue Organ Cult. PCTOC 2012, 110, 103–110. [Google Scholar] [CrossRef]
- Rehman, R.; Chaudhary, M.; Khawar, K.; Lu, G.; Mannan, A.; Zia, M. In Vitro Propagation of Caralluma Tuberculata and Evaluation of Antioxidant Potential. Biologia (Bratisl.) 2014, 69, 341–349. [Google Scholar] [CrossRef]
- Abdulhafiz, F.; Mohammed, A.; Kayat, F.; Zakaria, S.; Hamzah, Z.; Reddy Pamuru, R.; Gundala, P.B.; Reduan, M.F.H. Micropropagation of Alocasia Longiloba Miq and Comparative Antioxidant Properties of Ethanolic Extracts of the Field-Grown Plant, in Vitro Propagated and in Vitro-Derived Callus. Plants 2020, 9, 816. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant Callus: Mechanisms of Induction and Repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef] [Green Version]
- Fehér, A. Callus, Dedifferentiation, Totipotency, Somatic Embryogenesis: What These Terms Mean in the Era of Molecular Plant Biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef] [Green Version]
- Abdulhafiz, F. Plant Cell Culture Technologies: A Promising Alternatives to Produce High-Value Secondary Metabolites. Arab. J. Chem. 2022, 15, 104161. [Google Scholar] [CrossRef]
- Dal Toso, R.; Melandri, F. Plant Cell Culture Technology: A New Ingredient Source. CARE 2010, 28, 35–38. [Google Scholar]
- Fremont, F. Cell Culture: An Innovative Approach for Production of Plant Actives; Russell Publishing Ltd.: Brasted, UK, 2018. [Google Scholar]
- Gao, W.-Y.; Wang, J.; Li, J.; Wang, Q. Production of Biomass and Bioactive Compounds from Cell Suspension Cultures of Panax Quinquefolium L. and Glycyrrhiza Uralensis Fisch. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 143–164. [Google Scholar]
- Bagheri, F.; Tahvilian, R.; Karimi, N.; Chalabi, M.; Azami, M. Shikonin Production by Callus Culture of Onosma Bulbotrichom as Active Pharmaceutical Ingredient. Iran. J. Pharm. Res. IJPR 2018, 17, 495. [Google Scholar] [PubMed]
- Guo, S.; Man, S.; Gao, W.; Liu, H.; Zhang, L.; Xiao, P. Production of Flavonoids and Polysaccharide by Adding Elicitor in Different Cellular Cultivation Processes of Glycyrrhiza Uralensis Fisch. Acta Physiol. Plant. 2013, 35, 679–686. [Google Scholar] [CrossRef]
- Wang, Q.J.; Zheng, L.P.; Sima, Y.H.; Yuan, H.Y.; Wang, J.W. Methyl Jasmonate Stimulates 20-Hydroxyecdysone Production in Cell Suspension Cultures of’Achyranthes Bidentata’. Plant Omics 2013, 6, 116–120. [Google Scholar]
- Bimonte, M.; Tito, A.; Carola, A.; Barbulova, A.; Apone, F.; Colucci, G.; Cucchiara, M.; Hill, J. Dolichos Cell Culture Extract for Protection against UV Damage. Cosmet Toilet 2014, 129, 46–56. [Google Scholar]
- Imparato, G.; Casale, C.; Scamardella, S.; Urciuolo, F.; Bimonte, M.; Apone, F.; Colucci, G.; Netti, P. A Novel Engineered Dermis for in Vitro Photodamage Research. J. Tissue Eng. Regen. Med. 2017, 11, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Vertuani, S.; Beghelli, E.; Scalambra, E.; Malisardi, G.; Copetti, S.; Toso, R.D.; Baldisserotto, A.; Manfredini, S. Activity and Stability Studies of Verbascoside, a Novel Antioxidant, in Dermo-Cosmetic and Pharmaceutical Topical Formulations. Molecules 2011, 16, 7068–7080. [Google Scholar] [CrossRef]
- Bimonte, M.; Carola, A.; Tito, A.; Barbulova, A.; Carucci, F.; Apone, F. Coffea Bengalensis for Antiwrinkle and Skin Toning Applications. Cosmet. Toilet. 2011, 126, 644–650. [Google Scholar]
- Yue, W.; Ming, Q.; Lin, B.; Rahman, K.; Zheng, C.-J.; Han, T.; Qin, L. Medicinal Plant Cell Suspension Cultures: Pharmaceutical Applications and High-Yielding Strategies for the Desired Secondary Metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A Tool for Enriching the Bioactive Composition of Foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [Green Version]
- Vasconsuelo, A.; Boland, R. Molecular Aspects of the Early Stages of Elicitation of Secondary Metabolites in Plants. Plant Sci. 2007, 172, 861–875. [Google Scholar] [CrossRef]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A Biotechnological Tool for Enhanced Production of Secondary Metabolites in Hairy Root Cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usman, H.; Ullah, M.A.; Jan, H.; Siddiquah, A.; Drouet, S.; Anjum, S.; Giglioli-Guviarc’h, N.; Hano, C.; Abbasi, B.H. Interactive Effects of Wide-Spectrum Monochromatic Lights on Phytochemical Production, Antioxidant and Biological Activities of Solanum Xanthocarpum Callus Cultures. Molecules 2020, 25, 2201. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, R.; Docimo, T.; Graziani, G.; D’Amelia, V.; De Palma, M.; Cappetta, E.; Tucci, M. Abiotic Stresses Elicitation Potentiates the Productiveness of Cardoon Calli as Bio-Factories for Specialized Metabolites Production. Antioxidants 2022, 11, 1041. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Li, Q.; Tan, H.; Chen, J.; Xiao, Y.; Ma, R.; Gao, S.; Zerbe, P.; Chen, W.; Zhang, L. Gene-to-Metabolite Network for Biosynthesis of Lignans in MeJA-Elicited Isatis Indigotica Hairy Root Cultures. Front. Plant Sci. 2015, 6, 952. [Google Scholar] [CrossRef] [Green Version]
- Wen, T.; Hao, Y.-J.; An, X.-L.; Sun, H.-D.; Li, Y.-R.; Chen, X.; Piao, X.-C.; Lian, M.-L. Improvement of Bioactive Compound Accumulation in Cell Cultures of Orostachys Cartilaginous A. Bor. through Elicitation with Salicylic Acid and Effect of Cell Extract on Bioactive Activity. Ind. Crops Prod. 2019, 139, 111570. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Naik, P.M. Elicitor-Induced Production of Biomass and Pharmaceutical Phenolic Compounds in Cell Suspension Culture of Date Palm (Phoenix Dactylifera L.). Molecules 2020, 25, 4669. [Google Scholar] [CrossRef]
- Durán, M.D.L.; Zabala, M.E.A.; Londoño, G.A.C. Optimization of Flavonoid Production in Plant Cell Culture of Thevetia Peruviana Elicited with Methyl Jasmonate and Salicylic Acid. Braz. Arch. Biol. Technol. 2021, 64, e21210022. [Google Scholar] [CrossRef]
- Wongwicha, W.; Tanaka, H.; Shoyama, Y.; Putalun, W. Methyl Jasmonate Elicitation Enhances Glycyrrhizin Production in Glycyrrhiza Inflata Hairy Roots Cultures. Z. Für Naturforschung C 2011, 66, 423–428. [Google Scholar] [CrossRef]
- Shoja, A.A.; Çirak, C.; Ganjeali, A.; Cheniany, M. Stimulation of Phenolic Compounds Accumulation and Antioxidant Activity in in Vitro Culture of Salvia Tebesana Bunge in Response to Nano-TiO2 and Methyl Jasmonate Elicitors. Plant Cell Tissue Organ Cult. PCTOC 2022, 149, 423–440. [Google Scholar] [CrossRef]
- Pilaisangsuree, V.; Somboon, T.; Tonglairoum, P.; Keawracha, P.; Wongsa, T.; Kongbangkerd, A.; Limmongkon, A. Enhancement of Stilbene Compounds and Anti-Inflammatory Activity of Methyl Jasmonate and Cyclodextrin Elicited Peanut Hairy Root Culture. Plant Cell Tissue Organ Cult. PCTOC 2018, 132, 165–179. [Google Scholar] [CrossRef]
- Ayoola-Oresanya, I.O.; Sonibare, M.A.; Gueye, B.; Abberton, M.T.; Morlock, G.E. Elicitation of Antioxidant Metabolites in Musa Species in Vitro Shoot Culture Using Sucrose, Temperature and Jasmonic Acid. Plant Cell Tissue Organ Cult. PCTOC 2021, 146, 225–236. [Google Scholar] [CrossRef]
- Mosavat, N.; Golkar, P.; Yousefifard, M.; Javed, R. Modulation of Callus Growth and Secondary Metabolites in Different Thymus Species and Zataria Multiflora Micropropagated under ZnO Nanoparticles Stress. Biotechnol. Appl. Biochem. 2019, 66, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Mohammad, S.; Khan, M.A.; Raja, N.I.; Arif, M.; Kamil, A.; Mashwani, Z.-R. Silver Nanoparticles Elicited in Vitro Callus Cultures for Accumulation of Biomass and Secondary Metabolites in Caralluma Tuberculata. Artif. Cells Nanomedicine Biotechnol. 2019, 47, 715–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, I.-M.; Rajakumar, G.; Thiruvengadam, M. Effect of Silver Nanoparticles on Phenolic Compounds Production and Biological Activities in Hairy Root Cultures of Cucumis Anguria. Acta Biol. Hung. 2018, 69, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Javed, R.; Mohamed, A.; Yücesan, B.; Gürel, E.; Kausar, R.; Zia, M. CuO Nanoparticles Significantly Influence in Vitro Culture, Steviol Glycosides, and Antioxidant Activities of Stevia Rebaudiana Bertoni. Plant Cell Tissue Organ Cult. PCTOC 2017, 131, 611–620. [Google Scholar] [CrossRef]
- Zigoneanu, I.G.; Astete, C.E.; Sabliov, C.M. Nanoparticles with Entrapped α-Tocopherol: Synthesis, Characterization, and Controlled Release. Nanotechnology 2008, 19, 105606. [Google Scholar] [CrossRef] [PubMed]
- Królicka, A.; Lojkowska, E.; Staniszewska, I.; Malinski, E.; Szafranek, J. Identification of Secondary Metabolites in In Vitro Culture of Ammi Majus Treated with Elicitors. In Proceedings of the IV International Symposium on In Vitro Culture and Horticultural Breeding, Tampere, Finland, 2–7 July 2000; pp. 255–258. [Google Scholar]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, M.; Shujait Ali, S.; Khan, A.; Wei, D.-Q. Sustainable Production of Biomass and Industrially Important Secondary Metabolites in Cell Cultures of Selfheal (Prunella Vulgaris L.) Elicited by Silver and Gold Nanoparticles. Artif. Cells Nanomedicine Biotechnol. 2019, 47, 2553–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Q.; Hu, Z.; Tan, R.X.; Wu, J. Efficient Production and Recovery of Diterpenoid Tanshinones in Salvia Miltiorrhiza Hairy Root Cultures with in Situ Adsorption, Elicitation and Semi-Continuous Operation. J. Biotechnol. 2005, 119, 416–424. [Google Scholar] [CrossRef]
- Shakeran, Z.; Keyhanfar, M.; Ghanadian, M. Biotic Elicitation for Scopolamine Production by Hairy Root Cultures of Datura Metel. Mol. Biol. Res. Commun. 2017, 6, 169. [Google Scholar]
- Lu, M.; Wong, H.; Teng, W. Effects of Elicitation on the Production of Saponin in Cell Culture of Panax Ginseng. Plant Cell Rep. 2001, 20, 674–677. [Google Scholar] [CrossRef]
- Shams-Ardakani, M.; Hemmati, S.; Mohagheghzadeh, A. Effect of Elicitors on the Enhancement of Podophyllotoxin Biosynthesis in Suspension Cultures of Linum Album. DARU J. Pharm. Sci. 2005, 13, 56–60. [Google Scholar]
- Palazón, J.; Cusidó, R.M.; Bonfill, M.; Mallol, A.; Moyano, E.; Morales, C.; Piñol, M.T. Elicitation of Different Panax Ginseng Transformed Root Phenotypes for an Improved Ginsenoside Production. Plant Physiol. Biochem. 2003, 41, 1019–1025. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.-J.; Paek, K.-Y. Production of Secondary Metabolites from Cell and Organ Cultures: Strategies and Approaches for Biomass Improvement and Metabolite Accumulation. Plant Cell Tissue Organ Cult. PCTOC 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Javid, A.; Gampe, N.; Gelana, F.; György, Z. Enhancing the Accumulation of Rosavins in Rhodiola Rosea L. Plants Grown In Vitro by Precursor Feeding. Agronomy 2021, 11, 2531. [Google Scholar] [CrossRef]
- Ahmadian Chashmi, N.; Sharifi, M.; Behmanesh, M. Lignan Enhancement in Hairy Root Cultures of Linum Album Using Coniferaldehyde and Methylenedioxycinnamic Acid. Prep. Biochem. Biotechnol. 2016, 46, 454–460. [Google Scholar] [CrossRef]
- Karppinen, K.; Hokkanen, J.; Tolonen, A.; Mattila, S.; Hohtola, A. Biosynthesis of Hyperforin and Adhyperforin from Amino Acid Precursors in Shoot Cultures of Hypericum Perforatum. Phytochemistry 2007, 68, 1038–1045. [Google Scholar] [CrossRef]
- Jeong, C.-S.; Murthy, H.N.; Hahn, E.-J.; Paek, K.-Y. Improved Production of Ginsenosides in Suspension Cultures of Ginseng by Medium Replenishment Strategy. J. Biosci. Bioeng. 2008, 105, 288–291. [Google Scholar] [CrossRef]
- Wu, C.-H.; Murthy, H.N.; Hahn, E.-J.; Paek, K.-Y. Improved Production of Caffeic Acid Derivatives in Suspension Cultures Of Echinacea Purpurea by Medium Replenishment Strategy. Arch. Pharm. Res. 2007, 30, 945–949. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Mei, X. Enhanced Taxol Production and Release in Taxus ChinensisCell Suspension Cultures with Selected Organic Solvents and Sucrose Feeding. Biotechnol. Prog. 2001, 17, 89–94. [Google Scholar] [CrossRef]
- Yadav, D.; Tanveer, A.; Malviya, N.; Yadav, S. Overview and Principles of Bioengineering: The Drivers of Omics Technologies. In Omics Technologies and Bio-Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–23. [Google Scholar]
- Gonçalves, S.; Romano, A. Production of Plant Secondary Metabolites by Using Biotechnological Tools. Second. Metab.-Sources Appl. 2018, 5, 81–99. [Google Scholar]
- Vásquez, S.M.; Abascal, G.G.W.; Leal, C.E.; Cardineau, G.A.; Lara, S.G. Application of Metabolic Engineering to Enhance the Content of Alkaloids in Medicinal Plants. Metab. Eng. Commun. 2022, 14, e00194. [Google Scholar] [CrossRef] [PubMed]
- Verpoorte, R.; Contin, A.; Memelink, J. Biotechnology for the Production of Plant Secondary Metabolites. Phytochem. Rev. 2002, 1, 13–25. [Google Scholar] [CrossRef]
- Oksman-Caldentey, K.-M.; Arroo, R. Regulation of Tropane Alkaloid Metabolism in Plants and Plant Cell Cultures. In Metabolic Engineering of Plant Secondary Metabolism; Springer: Berlin/Heidelberg, Germany, 2000; pp. 253–281. [Google Scholar]
- Zhong, J.-J. Plant Cell Culture for Production of Paclitaxel and Other Taxanes. J. Biosci. Bioeng. 2002, 94, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, R.A. Secondary Metabolites of Medicinal Plants, 4 Volume Set: Ethnopharmacological Properties, Biological Activity and Production Strategies; John Wiley & Sons: Hoboken, NJ, USA, 2020; ISBN 3-527-34732-1. [Google Scholar]
- Galih, P.R.; Esyanti, R.R. Effect of Immobilization on Cell Growth and Alkaloid Contents in Cell-Aggregate Culture of Eurycoma Longifolia Jack. Int J Chem Env. Biol Sci 2014, 2, 90–93. [Google Scholar]
- Zhang, P.; Zhou, W.; Wang, P.; Wang, L.; Tang, M. Enhancement of Chitosanase Production by Cell Immobilization of Gongronella Sp. JG. Braz. J. Microbiol. 2013, 44, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Premjet, D.; Tachibana, S. Production of Podophyllotoxin by Immobilized Cell Cultures of Juniperus Chinensis. Pak. J Biol Sci 2004, 7, 1130–1134. [Google Scholar]
- Vanisree, M.; Lee, C.-Y.; Lo, S.-F.; Nalawade, S.M.; Lin, C.Y.; Tsay, H.-S. Studies on the Production of Some Important Secondary Metabolites from Medicinal Plants by Plant Tissue Cultures. Bot Bull Acad Sin 2004, 45, 1–22. [Google Scholar]
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.Z.; Saeed, M. Current Approaches toward Production of Secondary Plant Metabolites. J. Pharm. Bioallied Sci. 2012, 4, 10. [Google Scholar] [CrossRef]
- Malik, S.; Hossein Mirjalili, M.; Fett-Neto, A.G.; Mazzafera, P.; Bonfill, M. Living between Two Worlds: Two-Phase Culture Systems for Producing Plant Secondary Metabolites. Crit. Rev. Biotechnol. 2013, 33, 1–22. [Google Scholar] [CrossRef]
- Lee-Parsons, C.W.; Shuler, M.L. The Effect of Ajmalicine Spiking and Resin Addition Timing on the Production of Indole Alkaloids from Catharanthus Roseus Cell Cultures. Biotechnol. Bioeng. 2002, 79, 408–415. [Google Scholar] [CrossRef]
- Komaraiah, P.; Ramakrishna, S.; Reddanna, P.; Kishor, P.K. Enhanced Production of Plumbagin in Immobilized Cells of Plumbago Rosea by Elicitation and in Situ Adsorption. J. Biotechnol. 2003, 101, 181–187. [Google Scholar] [CrossRef]
- Klvana, M.; Legros, R.; Jolicoeur, M. In Situ Extraction Strategy Affects Benzophenanthridine Alkaloid Production Fluxes in Suspension Cultures of Eschscholtzia Californica. Biotechnol. Bioeng. 2005, 89, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.-B.; Zhang, W.; Ruan, C. Significantly Improved Taxuyunnanine C Production in Cell Suspension Cultures of Taxus Chinensis by Process Intensification of Repeated Elicitation, Sucrose Feeding, and in Situ Adsorption. World J. Microbiol. Biotechnol. 2011, 27, 2271–2279. [Google Scholar] [CrossRef]
- Chiang, L.; Abdullah, M.A. Enhanced Anthraquinones Production from Adsorbent-Treated Morinda Elliptica Cell Suspension Cultures in Production Medium Strategy. Process Biochem. 2007, 42, 757–763. [Google Scholar] [CrossRef]


| Cell Line | Plant Species | Plant Material | Bioactive Molecules (If Applicable) | Noticeable Effects | References |
|---|---|---|---|---|---|
| Fibroblasts | Centella asiatica | Callus derived from stolon segments | - | * Induction of the expression of antioxidant-encoding genes such as SOD, CAT, and GPX. * Inhibition of the transcription of MMP-7. | [48] |
| Chaenomeles japonica | Callus derived from leaf culture | Triterpenoids | * Stimulation of skin cell proliferation. * Notable antioxidant activity of callus-derived extract. | [49] | |
| Cinnamumum cassia | Bark of in vivo plant material | Trans-cinnamic acid | * Inhibition of intracellular accumulation of ROS. * Downregulation of metalloproteinases (MMP-1 and MMP-3). * Prevention of type I pro-collagen degradation in skin fibroblasts. * Activation of Nrf2 signaling mediated by AMPK, PKC, ROS, or CKII signaling cascades. * Positive regulation of γ-GCLC and HO-1 gene expression because of the activation of Nrf2. | [50] | |
| Citrus junos | Callus derived from leaf, flower, and seed cultures | p-hydroxycinnamoylmalic acid | * Inhibition of melanin biosynthesis. * Strong tyrosinase inhibitory effects. * Enhancement of collagen production. * Acceleration of wound healing process. | [51] | |
| Diplectria barbata | Leaves | - | * A dose-dependent increase in the expression of elastin and collagen genes. * An increase in cell survival, development, and differentiation-related proteins. * Decrease in MMP-3 expression. * Increase in the expression of HO-1 and Nrf2. | [52] | |
| Malus domesticus | Cell suspensions | - | * Reversal of senescence signs. * Stimulation of the expression of heme oxygenase 1. * Delay in cell apoptosis and senescence. | [53] | |
| Olea europaea | Leaves | - | * Inhibition of cell apoplastic death hypothetically through TrxR upregulation. * DNA damage suppression. * Inhibition of ROS production and accumulation. | [54] | |
| Panax ginseng | In vivo plant material | Ginsenoside RG3 | * Induction of the expression of extracellular matrix proteins, cell proliferation genes, and genes associated with growth. * Increase in the expression of antioxidant-encoding genes such as HO-1 and nuclear factor Nrf2. | [55] | |
| Piper cambodianum | In vivo plant material | - | * Increase in the expression of extracellular matrix genes such as elastin and collagen and hyaluronan synthase-2 in a dose-dependent manner. * Decrease in the expression of the MMP-3 gene. | [56] | |
| Pueraria candollei | Cell suspensions derived from stem segment culture | daidzein, genistin, genistein, khwakhurin, daidzin, and puerarin | * Stimulation of cell proliferation. * Strong antioxidant activity estimated through DPPH assay. | [57] | |
| Pyrus pyrifolia | Callus derived from cotyledon culture | - | * Repression of melanin biosynthesis. * Inhibitive action against tyrosinase. * Stimulation of the collagen biosynthesis in fibroblasts. * Acceleration of wound recovery process. | [58] | |
| Tiarella polyphylla | Callus derived from leaf, petiole, and stem segment cultures | Nicotiflorin, astragalin, quercitrin, myricitrin | * Protective effect against UV radiation. * Enhancement of type I pro-collagen amount. * Decrease in MMP-1 production through the repression of the expression of MMP genes. | [59] | |
| Woodfordia fruticose Kurz | Callus derived from young apical leaf culture | - | * Collagen and elastin biosynthesis production stimulation. * Positive regulation of the expression of collagen and elastic genes. * Delay in cell senescence. | [60] | |
| Keratinocytes | Camellia sinensis | In vivo plant leaves | - | * Accumulation of mRNA and proteins of phase I and II detoxifying enzymes, more likely HO-1. * Positive regulation of Nrf2-mediated pathway by triggering the phosphorylation of ERK and p38. * Downregulation of MMP-1. | [61] |
| Lindera erythrocarpa | Fruit | Lucidone | * Prevention of cell death due to UVA. * Prevention of mitochondria dysfunction, DNA degradation, and Bcl-2/Bax dysregulation. * Induction of antioxidant genes such as NQO-1, HO-A, and γ-GCLC through the transcriptional activation of Nrf2. * Reduction of ROS accumulation. | [62] | |
| Leontopodium alipnum | Callus derived from leaf culture | - | * Strong antioxidant activity in response to UV radiation. * Suppression of skin inflammation and wrinkles. * Improvement in skin elasticity, thickness, anti-periorbital wrinkles, and dermal density. * Upregulation of genes involved in cell apoptosis, keratinization, and cornification, forming skin barriers. | [63] | |
| Oryza sativa | Callus derived from seed culture | - | * Strong antioxidant activities of calli extracts. * Increase keratinocyte proliferation. * Strong anti-collagenase and anti-tyrosinase activities recorded. | [64] | |
| Phyllanthus emblica L. | In vivo plant material | - | * Elimination of excessive ROS. * Activation of antioxidant machinery (CAT). * Reduction in the release of cyt c. * Mitigation of the inflammatory mediator PGE2 and the inflammatory signals NF-κB and AP-1. * Inhibition of Akt overactivity. | [65] | |
| Polypodium vulgare | In vivo plant material | Shikimic acid Caffeoylquinic acid derivatives Epicatechin Catechin | * Activation of antioxidant systems. * Dose-dependent regulation of ROS production. | [66] | |
| Pyrus pyrifolia | Callus derived from young leaf culture | Uridine, guanosine, and adenosine | * Strong ABTS and DPPH antioxidant activity. * Notable collagen and elastin glycation inhibitory effect of callus extract. * Stimulation of cell proliferation. * Acceleration of wound healing process. * Stimulation of the production of pro-collagen. | [67] | |
| Syzygium samarangense | In vivo plant leaves | myricetin-3-O-α-rhamnoside 7,8,3′,4′-tetrahydroxy-3,5-dimethoxyflavone | * Reduction in intracellular ROS accumulation and carbonyl amounts. * Maintenance of intracellular GSH levels, even after exposure to toxic chemicals such as sodium arsenite. * Stimulation of Nrf-2 transcription factor translocation to the nucleus, which induces the expression of Mn-SOD3 and HO-1 genes. * Inhibition of IκB-α degradation. | [68] | |
| Zingiber zerumbet | Rhizomes | Zerumbone | * Prevention of lactate dehydrogenase release in a dose-proportional way. * Reduction in DNA alteration, ROS synthesis, Bax/Bcl-2 ratio dysregulation, and DNA single-strand breaks. * Transcriptional activation of Nrf2 through the action of p38 MAPL, PKC, and PI3K/AKT cascades. * Induction of antioxidant machinery, especially HO-1 and γ-GCLC. | [69] | |
| Melanoma cells | Panax ginseng | Hairy roots derived from cotyledon and hypocotyl cultures | - | * Inhibition of the accumulation of melanin. * Anti-tyrosinase and anti-melanogenic activity of plant extract. * Enhancement of the production of anthocyanin. | [70] |
| Epidermal melanocyte | Brassica rapa | Hairy roots derived from cotyledon culture | - | * Strong inhibitory action against tyrosinase. * Inhibition of melanin production. * Inhibition of AMPc levels. * Repression of the microphtamia-associated transcription factor (MITF) | [71] |
| Cell Line | Plant Species | Plant Material | Bioactive Molecules (If Applicable) | Noticeable Effects | References |
|---|---|---|---|---|---|
| Fibroblasts | Euterpe oleracea Martius | Fruits | Malvidin and cyanidin derivatives | * Prevention of lipid peroxidation * Reduction in the adverse effects of UVA-induced stress. * Interference in ROS generation. * Maintenance of GSH level at normal. | [72] |
| Nicotiana tabaccum | Cell suspension derived from leaf segment culture | - | * Low ROS production. * Strong antioxidant capacity of the extract. * Upregulation of genes involved in DNA protection such as GADD45, SIRT-1, and SIRT-6. * Repression of the expression of MMP genes. | [73] | |
| Thymus vulgaris | In vivo plant material | - | * Prevention of collagen degradation through the inhibition of MMP-1 expression. * Inhibition of ROS accumulation. * Increase in DLD production, known as a cellular defense against oxidative stress. * Inhibition of MAPKs and AP-1 signaling pathways. | [74] | |
| Solanum lycopersicum | Cell suspensions derived from leaf culture | Rutin, chlorogenic acid, coumaric acid, ferulic acid, and glucosides | * Lower production of ROS. * Strong expression of antioxidant genes. * Reduction in nuclear DNA damage. * Decrease in the expression of MMP genes. | [75] | |
| Keratinocytes | Solanum lycopersicum | Cell suspensions derived from leaf culture | Rutin, chlorogenic acid, coumaric acid, ferulic acid, and glucosides | * Lower production of ROS. * Strong expression of antioxidant genes. * Reduction in nuclear DNA damage. * Decrease in the expression of MMP genes. | [75] |
| Melanoma cells | Brassica rapa | Hairy roots derived from cotyledon culture | - | * Strong inhibitory action against tyrosinase. * Inhibition of melanin production. * Inhibition of AMPc levels. * Repression of the microphthalmia-associated transcription factor (MITF). | [71] |
| Elicitor Type (Abiotic/Biotic/Combined) | Elicitors | Culture Type | Plant Species | Bioactive Compounds Identified in the PCC System | References |
|---|---|---|---|---|---|
| Abiotic | Salicylic acid | Cell suspensions | Orostachys cartilaginous | quercetin, quercetin-3-O-glucose kaempferide, kaempferol-3-rutinoside, and epicatechin gallate | [116] |
| Salicylic acid | Cell suspensions | Pueraria candollei | Khwakhurin, daidzein, puerarin, and genistin | [57] | |
| Salicylic acid | Cell suspensions | Phoenix dactylifera L. | Catechin, apigenin, kaempferol, caffeic acid | [117] | |
| Salicylic acid and Methyl jasmonate | Cell suspensions | Thevetia peruviana | N/A | [118] | |
| Methyl jasmonate | Hairy roots | Glycyrrhiza inflata | Glycyrrhizin | [119] | |
| Methyl jasmonate and titanium oxide nanoparticles | Callus | Salvia tebesana | O-diphenols, avonol phenolic acid, avonoid, and avone | [120] | |
| Methyl jasmonate and cyclodextrin | Hairy roots | Arachis hypogea | Stilbene | [121] | |
| Jasmonic acid and sucrose | In vitro shoot culture | Musa sp. | N/A | [122] | |
| Zinc oxide nanoparticles | Callus | T. vulgaris, T.daenensis, T. kotschyanus and Zataria multiflora | Thymol and carvacrol | [123] | |
| Silver nanoparticles | Callus | Caralluma tuberculata | N/A | [124] | |
| Silver nitrate and silver nanoparticles | Hairy roots | Cucumis anguria L. | Coumaric, p-coumaric, vanillic acid, ferulic, caffeic, protocathechuicand t-cinnamic | [125] | |
| Copper oxide nanoparticles | In vitro shoot culture | Stevia rebaudiana | Steviol glycosides | [126] | |
| Titanium dioxide nanoparticles and silicon dioxide | Callus | Argania spinosa | α-tocopherol | [127] | |
| Silicon oxide | Callus | Ammi majus | umbelliferone | [128] | |
| Silicon oxide | Hairy roots | Ammi majus | Umbelliferone | [128] | |
| Gold, silver, and NAA | Callus | Prunella vulgaris | N/A | [129] | |
| NaCl | Callus | Cynara cardunculus | N/A | [114] | |
| Blue light | Callus | Solanum xanthocarpum | ethyl-caffeate, esculetin, caffeic acid, and scopoletin | [113] | |
| Biotic | Yeast extract | Hairy roots | Salvia miltiorrhiza | Diterpenoid tanshinones | [130] |
| Bacillus cereus, Staphylococcus aureus | Hairy roots | Datura metel | Scopolamine | [131] | |
| Combined | Methyl jasmonate and chitosan | Cell suspensions | Pueraria candollei | khwakhurin, daidzein, puerarin, and genistin | [57] |
| Methyl jasmonate and yeast extract | Cell suspensions | Panax ginseng | Saponin | [132] | |
| Yeast extract & Ag+, Pb2+ and Cd2+ elicitors | Cell suspensions | Linum album | Podophyllotoxin | [133] | |
| Methyl jasmonate, chitosan, and vanadyl sulfate | Hairy roots | Panax ginseng | ginsenoside | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzroud, S.; El Maaiden, E.; Sobeh, M.; Merghoub, N.; Boukcim, H.; Kouisni, L.; El Kharrassi, Y. Biotechnological Approaches to Producing Natural Antioxidants: Anti-Ageing and Skin Longevity Prospects. Int. J. Mol. Sci. 2023, 24, 1397. https://doi.org/10.3390/ijms24021397
Bouzroud S, El Maaiden E, Sobeh M, Merghoub N, Boukcim H, Kouisni L, El Kharrassi Y. Biotechnological Approaches to Producing Natural Antioxidants: Anti-Ageing and Skin Longevity Prospects. International Journal of Molecular Sciences. 2023; 24(2):1397. https://doi.org/10.3390/ijms24021397
Chicago/Turabian StyleBouzroud, Sarah, Ezzouhra El Maaiden, Mansour Sobeh, Nawal Merghoub, Hassan Boukcim, Lamfeddal Kouisni, and Youssef El Kharrassi. 2023. "Biotechnological Approaches to Producing Natural Antioxidants: Anti-Ageing and Skin Longevity Prospects" International Journal of Molecular Sciences 24, no. 2: 1397. https://doi.org/10.3390/ijms24021397