Neuron-Glial Antigen 2 Participates in Liver Fibrosis via Regulating the Differentiation of Bone Marrow Mesenchymal Stem Cell to Myofibroblast
Abstract
:1. Introduction
2. Results
2.1. The Dynamic Changes in NG2 Expression in the Fibrotic Livers of Patients and Multiple Mouse Models
2.2. The Correlation between CSPG4 and Fibrosis Markers in Human and Mouse Liver
2.3. The High Expression of NG2 on BMSC-Derived MF in Fibrotic Liver
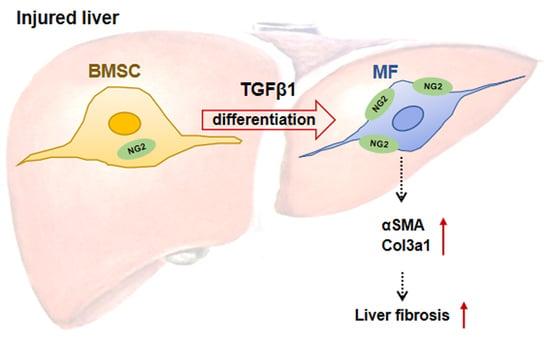
2.4. The NG2 Expression in TGFβ1-Induced BMSC Differentiation to MF
2.5. The Involvement of NG2 in TGFβ1-Induced BMSC Differentiation to MF
2.6. The Participation of NG2 in Liver Fibrosis In Vivo
3. Discussion
4. Materials and Methods
4.1. Mouse Models
4.2. Human Liver Specimens
4.3. BMSC Isolation and Culture
4.4. qRT-PCR
4.5. Immunofluorescence Staining
4.6. Western Blot Analysis
4.7. Quantitative Analysis of Liver Fibrosis, Steatosis and Inflammation
4.8. ALT and AST Measurement
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, F.; Wong, N.K.; He, J.; Zhang, R.; Sun, R.; Xu, Y.; Liu, Y.; Li, W.; Koike, K.; et al. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J. Hepatol. 2019, 71, 212–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parola, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Aspects Med. 2019, 65, 37–55. [Google Scholar] [CrossRef]
- Russo, F.P.; Alison, M.R.; Bigger, B.W.; Amofah, E.; Florou, A.; Amin, F.; Bou-Gharios, G.; Jeffery, R.; Iredale, J.P.; Forbes, S.J. The bone marrow functionally contributes to liver fibrosis. Gastroenterology 2006, 130, 1807–1821. [Google Scholar] [CrossRef]
- Forbes, S.J.; Russo, F.P.; Rey, V.; Burra, P.; Rugge, M.; Wright, N.A.; Alison, M.R. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology 2004, 126, 955–963. [Google Scholar] [CrossRef]
- Yang, L.; Chang, N.; Liu, X.; Han, Z.; Zhu, T.; Li, C.; Yang, L.; Li, L. Bone marrow-derived mesenchymal stem cells differentiate to hepatic myofibroblasts by transforming growth factor-beta1 via sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis. Am. J. Pathol. 2012, 181, 85–97. [Google Scholar] [CrossRef]
- Li, C.; Kong, Y.; Wang, H.; Wang, S.; Yu, H.; Liu, X.; Yang, L.; Jiang, X.; Li, L.; Li, L. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J. Hepatol. 2009, 50, 1174–1183. [Google Scholar] [CrossRef]
- Li, C.; Jiang, X.; Yang, L.; Liu, X.; Yue, S.; Li, L. Involvement of sphingosine 1-phosphate (SIP)/S1P3 signaling in cholestasis-induced liver fibrosis. Am. J. Pathol. 2009, 175, 1464–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goss, G.; Rognoni, E.; Salameti, V.; Watt, F.M. Distinct Fibroblast Lineages Give Rise to NG2+ Pericyte Populations in Mouse Skin Development and Repair. Front. Cell Dev. Biol. 2021, 9, 675080. [Google Scholar] [CrossRef] [PubMed]
- Stallcup, W.B. NG2 Proteoglycan Enhances Brain Tumor Progression by Promoting Beta-1 Integrin Activation in both Cis and Trans Orientations. Cancers (Basel) 2017, 9, 31. [Google Scholar] [CrossRef]
- Campoli, M.R.; Chang, C.C.; Kageshita, T.; Wang, X.; McCarthy, J.B.; Ferrone, S. Human high molecular weight-melanoma-associated antigen (HMW-MAA): A melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit. Rev. Immunol. 2004, 24, 267–296. [Google Scholar] [CrossRef] [PubMed]
- Balabanov, R.; Dore-Duffy, P. Role of the CNS microvascular pericyte in the blood-brain barrier. J. Neurosci. Res. 1998, 53, 637–644. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Stallcup, W.B. The NG2 antigen, a putative lineage marker: Immunofluorescent localization in primary cultures of rat brain. Dev. Biol. 1981, 83, 154–165. [Google Scholar] [CrossRef] [PubMed]
- De Vrij, F.M.; Bouwkamp, C.G.; Gunhanlar, N.; Shpak, G.; Lendemeijer, B.; Baghdadi, M.; Gopalakrishna, S.; Ghazvini, M.; Li, T.M.; Quadri, M.; et al. Candidate CSPG4 mutations and induced pluripotent stem cell modeling implicate oligodendrocyte progenitor cell dysfunction in familial schizophrenia. Mol. Psychiatry 2019, 24, 757–771. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.H.; Cossu, G.; Herlyn, M.; Bell, J.R.; Steplewski, Z.; Koprowski, H. Isolation and chemical characterization of a melanoma-associated proteoglycan antigen. Arch. Biochem. Biophys. 1983, 225, 370–383. [Google Scholar] [CrossRef]
- Chekenya, M.; Rooprai, H.K.; Davies, D.; Levine, J.M.; Butt, A.M.; Pilkington, G.J. The NG2 chondroitin sulfate proteoglycan: Role in malignant progression of human brain tumours. Int. J. Dev. Neurosci. 1999, 17, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Rivera, Z.; Ferrone, S.; Wang, X.; Jube, S.; Yang, H.; Pass, H.I.; Kanodia, S.; Gaudino, G.; Carbone, M. CSPG4 as a target of antibody-based immunotherapy for malignant mesothelioma. Clin. Cancer Res. 2012, 18, 5352–5363. [Google Scholar] [CrossRef] [Green Version]
- Nicolosi, P.A.; Dallatomasina, A.; Perris, R. Theranostic impact of NG2/CSPG4 proteoglycan in cancer. Theranostics 2015, 5, 530–544. [Google Scholar] [CrossRef] [Green Version]
- Egan, C.E.; Stefanova, D.; Ahmed, A.; Raja, V.J.; Thiesmeyer, J.W.; Chen, K.J.; Greenberg, J.A.; Zhang, T.; He, B.; Finnerty, B.M.; et al. CSPG4 Is a Potential Therapeutic Target in Anaplastic Thyroid Cancer. Thyroid 2021, 31, 1481–1493. [Google Scholar] [CrossRef]
- Wang, X.; Osada, T.; Wang, Y.; Yu, L.; Sakakura, K.; Katayama, A.; McCarthy, J.B.; Brufsky, A.; Chivukula, M.; Khoury, T.; et al. CSPG4 protein as a new target for the antibody-based immunotherapy of triple-negative breast cancer. J. Natl. Cancer Inst. 2010, 102, 1496–1512. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, Z.; Hu, D.; Yan, M.; Sun, J.; Lai, J.; Bai, L. Immunotherapeutic Targeting of NG2/CSPG4 in Solid Organ Cancers. Vaccines 2022, 10, 1023. [Google Scholar] [CrossRef]
- Svendsen, A.; Verhoeff, J.J.; Immervoll, H.; Brogger, J.C.; Kmiecik, J.; Poli, A.; Netland, I.A.; Prestegarden, L.; Planaguma, J.; Torsvik, A.; et al. Expression of the progenitor marker NG2/CSPG4 predicts poor survival and resistance to ionising radiation in glioblastoma. Acta Neuropathol. 2011, 122, 495–510. [Google Scholar] [CrossRef] [Green Version]
- You, W.K.; Yotsumoto, F.; Sakimura, K.; Adams, R.H.; Stallcup, W.B. NG2 proteoglycan promotes tumor vascularization via integrin-dependent effects on pericyte function. Angiogenesis 2014, 17, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Dong, C.; Yang, J.; Yang, L.; Chang, N.; Qi, C.; Li, L. MicroRNA-26b-5p Inhibits Mouse Liver Fibrogenesis and Angiogenesis by Targeting PDGF Receptor-Beta. Mol. Ther. Nucleic Acids 2019, 16, 206–217. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander, S.B.; Mostafa, I.; Bugianesi, E.; Wai-Sun, W.V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M. Non-alcoholic fatty liver disease-A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, S.J.; Gupta, S.; Dhawan, A. Cell therapy for liver disease: From liver transplantation to cell factory. J. Hepatol. 2015, 62 (Suppl. 1), S157–S169. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Tian, L.; Zhang, Z.; Zhou, X.; Ji, X.; Liu, F.; Dong, C.; Hou, L.; Zhao, X.; Chang, N.; et al. Cannabinoid Receptor 1/miR-30b-5p Axis Governs Macrophage NLRP3 Expression and Inflammasome Activation in Liver Inflammatory Disease. Mol. Ther. Nucleic Acids 2020, 20, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.L.; Sun, J.; Lai, J.J.; Jiang, Y.; Bai, L.H.; Zhang, L.D. Neuron-glial antigen 2 overexpression in hepatocellular carcinoma predicts poor prognosis. World J. Gastroenterol. 2015, 21, 6649–6659. [Google Scholar] [CrossRef] [PubMed]
- Dohan, O.; Baloch, Z.; Banrevi, Z.; Livolsi, V.; Carrasco, N. Rapid communication: Predominant intracellular overexpression of the Na(+)/I(-) symporter (NIS) in a large sampling of thyroid cancer cases. J. Clin. Endocrinol. Metab. 2001, 86, 2697–2700. [Google Scholar] [CrossRef]
- Yamazaki, T.; Mukouyama, Y.S. Tissue Specific Origin, Development, and Pathological Perspectives of Pericytes. Front Cardiovasc Med 2018, 5, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, A.W.; Hindle, P.; Murray, I.R.; West, C.C.; Tawonsawatruk, T.; Shen, J.; Asatrian, G.; Zhang, X.; Nguyen, V.; Simpson, A.H.; et al. Pericytes for the treatment of orthopedic conditions. Pharmacol. Ther. 2017, 171, 93–103. [Google Scholar] [CrossRef]
- Trost, A.; Lange, S.; Schroedl, F.; Bruckner, D.; Motloch, K.A.; Bogner, B.; Kaser-Eichberger, A.; Strohmaier, C.; Runge, C.; Aigner, L.; et al. Brain and Retinal Pericytes: Origin, Function and Role. Front. Cell. Neurosci. 2016, 10, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sa, D.B.D.; Casamitjana, J.; Crisan, M. Pericytes, integral components of adult hematopoietic stem cell niches. Pharmacol Ther 2017, 171, 104–113. [Google Scholar]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol. Neurodegener. 2010, 5, 32. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhang, H.; Dong, C.; Yue, W.; Xue, R.; Liu, F.; Yang, L.; Li, L. Neuron-Glial Antigen 2 Participates in Liver Fibrosis via Regulating the Differentiation of Bone Marrow Mesenchymal Stem Cell to Myofibroblast. Int. J. Mol. Sci. 2023, 24, 1177. https://doi.org/10.3390/ijms24021177
Yang L, Zhang H, Dong C, Yue W, Xue R, Liu F, Yang L, Li L. Neuron-Glial Antigen 2 Participates in Liver Fibrosis via Regulating the Differentiation of Bone Marrow Mesenchymal Stem Cell to Myofibroblast. International Journal of Molecular Sciences. 2023; 24(2):1177. https://doi.org/10.3390/ijms24021177
Chicago/Turabian StyleYang, Le, Hang Zhang, Chengbin Dong, Wenhui Yue, Renmin Xue, Fuquan Liu, Lin Yang, and Liying Li. 2023. "Neuron-Glial Antigen 2 Participates in Liver Fibrosis via Regulating the Differentiation of Bone Marrow Mesenchymal Stem Cell to Myofibroblast" International Journal of Molecular Sciences 24, no. 2: 1177. https://doi.org/10.3390/ijms24021177