Defining the Molecular Mechanisms of the Relaxant Action of Adiponectin on Murine Gastric Fundus Smooth Muscle: Potential Translational Perspectives on Eating Disorder Management
Abstract
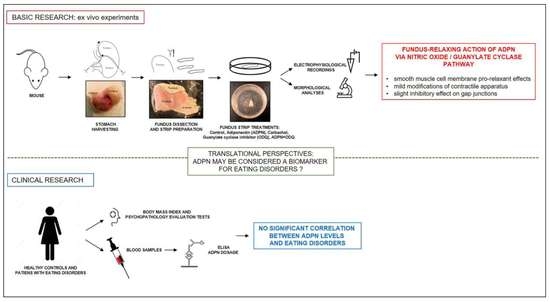
:1. Introduction
2. Results
2.1. Basic Research
2.1.1. ADPN Relaxant Effects on SMCs of the Murine Gastric Fundus Involve GC Activity
2.1.2. ADPN Slightly Reduces Gap Junction Function and Cx43 Expression in Gastric SMCs by GC Activation
2.2. Clinical Study
3. Discussion
Study Limitations and Future Perspectives
4. Conclusions
5. Materials and Methods
5.1. Basic Research: Studies on the Isolated Murine Gastric Fundus
5.1.1. Animals, Sample Preparations, and Treatments
5.1.2. Morphological Analysis
Hematoxylin and Eosin Staining (H&E)
Transmission Electron Microscopy (TEM)
Confocal Laser Scanning Microscopy
5.1.3. Electrophysiological Recordings
5.2. Clinical Research
5.2.1. Participants
5.2.2. Assessment and Measures
5.3. Data Analyses and Statistical Tests
5.3.1. Statistical Analysis of Electrophysiological Data
5.3.2. Statistical Analysis of Morphological Data
5.3.3. Statistical Analysis of Clinical Data
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADPN | Adiponectin |
| AMPK | 5′ AMP-activated protein kinase |
| AN | Anorexia Nervosa |
| a.u. | arbitrary units |
| BED | Binge-Eating Disorder |
| BN | Bulimia Nervosa |
| BMI | Body mass index |
| CCh | Carbachol |
| cGMP | Cyclic guanosine monophosphate |
| Cm | Cell linear capacitance |
| CTRL | Control |
| CV | Coefficient of variation |
| Cx43 | Connexin43 |
| ED | Eating disorders |
| EDE-Q | Eating Disorder Examination Questionnaire |
| EES | Emotional Eating Scale |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| GC | Guanylate cyclase |
| GI | Gastrointestinal |
| GJ | Gap junctions |
| H&E | Hematoxylin and eosin |
| HC | Healthy subjects |
| HP | Holding potential |
| ICC | Interstitial cells of Cajal |
| Ij | Trans-junctional currents |
| Ij,inst | Instantaneous trans-junctional currents |
| Ij,ss | Steady-state trans-junctional currents |
| IK | Voltage-dependent delayed rectifier K+ currents |
| nNOS | Neuronal nitric oxide synthase |
| NO | Nitric oxide |
| ODQ | 1H-[1,2,4] oxadiazolo[4,3-α]-quinoxalin-1-one |
| PBS | Phosphate-buffered saline |
| PDE | Phosphodiesterase |
| PI | Propidium iodide |
| RMP | Resting membrane potential |
| ROI | Regions of interest |
| SCL-90-R GSI | Symptom Checklist-90 Revised Global Severity Index |
| SD | Standard deviation |
| sma | Smooth muscle actin |
| SMC | Smooth muscle cell |
| TEM | Transmission electron microscopy |
| TTX | Tetrodotoxin |
| Vj | Trans-junctional voltage |
References
- Galley, J.C.; Singh, S.; Awata, W.M.C.; Alves, J.V.; Bruder-Nascimento, T. Adipokines: Deciphering the cardiovascular signature of adipose tissue. Biochem. Pharm. 2022, 206, 115324. [Google Scholar] [CrossRef] [PubMed]
- Suyama, S.; Maekawa, F.; Maejima, Y.; Kubota, N.; Kadowaki, T.; Yada, T. Glucose level determines excitatory or inhibitory effects of adiponectin on arcuate POMC neuron activity and feeding. Sci. Rep. 2016, 6, 30796. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Gao, Y.; Yao, T.; Huang, Y.; He, Z.; Kong, X.; Yu, K.J.; Wang, R.T.; Guo, H.; Yan, J.; et al. Adiponectin potentiates the acute effects of leptin in arcuate Pomc neurons. Mol. Metab. 2016, 5, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Suyama, S.; Lei, W.; Kubota, N.; Kadowaki, T.; Yada, T. Adiponectin at physiological level glucose-independently enhances inhibitory postsynaptic current onto NPY neurons in the hypothalamic arcuate nucleus. Neuropeptides 2017, 65, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Idrizaj, E.; Garella, R.; Nistri, S.; Dell’Accio, A.; Cassioli, E.; Rossi, E.; Castellini, G.; Ricca, V.; Squecco, R.; Baccari, M.C. Adiponectin Exerts Peripheral Inhibitory Effects on the Mouse Gastric Smooth Muscle through the AMPK Pathway. Int. J. Mol. Sci. 2020, 21, 9617. [Google Scholar] [CrossRef]
- Tack, J.; Demedts, I.; Meulemans, A.; Schuurkes, J.; Janssens, J. Role of nitric oxide in the gastric accommodation reflex and in meal induced satiety in humans. Gut 2002, 51, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Aljafary, M.A.; Al-Suhaimi, E.A. Adiponectin System (Rescue Hormone): The Missing Link between Metabolic and Cardiovascular Diseases. Pharmaceutics 2022, 14, 1430. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, H.; Yin, C.; Lan, X.; Wu, L.; Du, X.; Griffiths, H.R.; Gao, D. Adipokines, Hepatokines and Myokines: Focus on Their Role and Molecular Mechanisms in Adipose Tissue Inflammation. Front. Endocrinol. 2022, 13, 873699. [Google Scholar] [CrossRef]
- Tyszkiewicz-Nwafor, M.; Jowik, K.; Paszynska, E.; Dutkiewicz, A.; Słopien, A.; Dmitrzak-Weglarz, M. Expression of immune-related proteins and their association with neuropeptides in adolescent patients with anorexia nervosa. Neuropeptides 2022, 91, 102214. [Google Scholar] [CrossRef]
- Nobis, S.; Morin, A.; Achamrah, N.; Belmonte, L.; Legrand, R.; Chan, P.; do Rego, J.L.; Vaudry, D.; Gourcerol, G.; Déchelotte, P.; et al. Delayed gastric emptying and altered antrum protein metabolism during activity-based anorexia. Neurogastroenterol. Motil. 2018, 30, e13305. [Google Scholar] [CrossRef]
- Nguyen, T.M.D. Adiponectin: Role in Physiology and Pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Kindt, S.; Tack, J. Impaired gastric accommodation and its role in dyspepsia. Gut 2006, 55, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.E.; Coley, B.; Crandall, W.; Di Lorenzo, C.; Bravender, T. Effect of nutritional rehabilitation on gastric motility and somatization in adolescents with anorexia. J. Pediat. 2013, 163, 867–872.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azpiroz, F.; Malagelada, J.R. Vagally mediated gastric relaxation induced by intestinal nutrients in the dog. Am. J. Physiol. 1986, 251, G727–G735. [Google Scholar] [CrossRef] [PubMed]
- Coulie, B.; Tack, J.; Sifrim, D.; Andrioli, A.; Janssens, J. Role of nitric oxide in fasting gastric fundus tone and in 5-HT1 receptor-mediated relaxation of gastric fundus. Am. J. Physiol. 1999, 276, G373–G377. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.M.; Zembowicz, A.; Sessa, W.C.; Vane, J.R. Nitroxergic nerves mediate vagally induced relaxation in the isolated stomach of the guinea pig. Proc. Natl. Acad. Sci. USA 1991, 88, 11490–11494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuiken, S.D.; Vergeer, M.; Heisterkamp, S.H.; Tytgat, G.N.; Boeckxstaens, G.E. Role of nitric oxide in gastric motor and sensory functions in healthy subjects. Gut 2002, 51, 212–218. [Google Scholar] [CrossRef]
- Bódi, N.; Szalai, Z.; Bagyánszki, M. Nitrergic Enteric Neurons in Health and Disease-Focus on Animal Models. Int. J. Mol. Sci. 2019, 20, 2003. [Google Scholar] [CrossRef] [Green Version]
- Lies, B.; Gil, V.; Groneberg, D.; Seidler, B.; Saur, D.; Wischmeyer, E.; Jiménez, M.; Friebe, A. Interstitial cells of Cajal mediate nitrergic inhibitory neurotransmission in the murine gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 307, G98–G106. [Google Scholar] [CrossRef] [Green Version]
- Squecco, R.; Garella, R.; Idrizaj, E.; Nistri, S.; Francini, F.; Baccari, M.C. Relaxin Affects Smooth Muscle Biophysical Properties and Mechanical Activity of the Female Mouse Colon. Endocrinology 2015, 156, 4398–4410. [Google Scholar] [CrossRef]
- Idrizaj, E.; Garella, R.; Castellini, G.; Mohr, H.; Pellegata, N.S.; Francini, F.; Ricca, V.; Squecco, R.; Baccari, M.C. Adiponectin affects the mechanical responses in strips from the mouse gastric fundus. World J. Gastroenterol. 2018, 24, 4028–4035. [Google Scholar] [CrossRef] [PubMed]
- Idrizaj, E.; Garella, R.; Castellini, G.; Francini, F.; Ricca, V.; Baccari, M.C.; Squecco, R. Adiponectin Decreases Gastric Smooth Muscle Cell Excitability in Mice. Front. Physiol. 2019, 10, 1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassioli, E.; Rossi, E.; Squecco, R.; Baccari, M.C.; Maggi, M.; Vignozzi, L.; Comeglio, P.; Gironi, V.; Lelli, L.; Rotella, F.; et al. Reward and psychopathological correlates of eating disorders: The explanatory role of leptin. Psychiatry Res. 2020, 290, 113071. [Google Scholar] [CrossRef] [PubMed]
- Modzelewska, B.; Drygalski, K.; Hady, H.R.; Kiełczewska, A.; Chomentowski, A.; Koryciński, K.; Głuszyńska, P.; Kleszczewski, T. Resveratrol Relaxes Human Gastric Smooth Muscles Through High Conductance Calcium-Activated Potassium Channel in a Nitric Oxide-independent Manner. Front. Pharmacol. 2022, 13, 823887. [Google Scholar] [CrossRef]
- Idrizaj, E.; Garella, R.; Squecco, R.; Baccari, M.C. Can adiponectin have an additional effect on the regulation of food intake by inducing gastric motor changes? World J. Gastroenterol. 2020, 26, 2472–2478. [Google Scholar] [CrossRef]
- Mediavilla, C. Bidirectional gut-brain communication: A role for orexin-A. Neurochem. Int. 2020, 141, 104882. [Google Scholar] [CrossRef]
- Gaetani, S.; Romano, A.; Provensi, G.; Ricca, V.; Lutz, T.; Passani, M.B. Eating disorders: From bench to bedside and back. J. Neurochem. 2016, 139, 691–699. [Google Scholar] [CrossRef] [Green Version]
- Matias, I.; Bisogno, T.; Di Marzo, V. Endogenous cannabinoids in the brain and peripheral tissues: Regulation of their levels and control of food intake. Int. J. Obes. 2006, 30 (Suppl. 1), S7–S12. [Google Scholar] [CrossRef] [Green Version]
- Diéguez, C.; Vazquez, M.J.; Romero, A.; López, M.; Nogueiras, R. Hypothalamic control of lipid metabolism: Focus on leptin, ghrelin and melanocortins. Neuroendocrinology 2011, 94, 1–11. [Google Scholar] [CrossRef]
- Ghourab, S.; Beale, K.E.; Semjonous, N.M.; Simpson, K.A.; Martin, N.M.; Ghatei, M.A.; Bloom, S.R.; Smith, K.L. Intracerebroventricular administration of vasoactive intestinal peptide inhibits food intake. Regul. Pept. 2011, 172, 8–15. [Google Scholar] [CrossRef]
- Lateef, D.M.; Washington, M.C.; Sayegh, A.I. The short term satiety peptide cholecystokinin reduces meal size and prolongs intermeal interval. Peptides 2011, 32, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.A.; Bloom, S.R. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology 2012, 63, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Tsuneki, H.; Wada, T.; Sasaoka, T. Role of orexin in the central regulation of glucose and energy homeostasis. Endocr. J. 2012, 59, 365–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, S.C.; Begg, D.P. Food for Thought: Revisiting the Complexity of Food Intake. Cell Metab. 2015, 22, 348–351. [Google Scholar] [CrossRef] [Green Version]
- Tack, J.; Verbeure, W.; Mori, H.; Schol, J.; Van den Houte, K.; Huang, I.H.; Balsiger, L.; Broeders, B.; Colomier, E.; Scarpellini, E.; et al. The gastrointestinal tract in hunger and satiety signalling. United Eur. Gastroenterol. J. 2021, 9, 727–734. [Google Scholar] [CrossRef]
- Hajishafiee, M.; Bitarafan, V.; Feinle-Bisset, C. Gastrointestinal Sensing of Meal-Related Signals in Humans, and Dysregulations in Eating-Related Disorders. Nutrients 2019, 11, 1298. [Google Scholar] [CrossRef] [Green Version]
- Murthy, K.S.; Grider, J.R.; Makhlouf, G.M. InsP3-dependent Ca2+ mobilization in circular but not longitudinal muscle cells of intestine. Am. J. Physiol. 1991, 261, G937–G944. [Google Scholar] [CrossRef]
- Cordaillat, M.; Fort, A.; Virsolvy, A.; Elghozi, J.L.; Richard, S.; Jover, B. Nitric oxide pathway counteracts enhanced contraction to membrane depolarization in aortic rings of rats on high-sodium diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1557–R1562. [Google Scholar] [CrossRef] [Green Version]
- Alioua, A.; Tanaka, Y.; Wallner, M.; Hofmann, F.; Ruth, P.; Meera, P.; Toro, L. The large conductance, voltage-dependent, and calcium-sensitive K+ channel, Hslo, is a target of cGMP-dependent protein kinase phosphorylation in vivo. J. Biol. Chem. 1998, 273, 32950–32956. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, F. The cGMP system: Components and function. Biol. Chem. 2020, 401, 447–469. [Google Scholar] [CrossRef]
- Wanstall, J.C.; Homer, K.L.; Doggrell, S.A. Evidence for, and importance of, cGMP-independent mechanisms with NO and NO donors on blood vessels and platelets. Cur. Vasc. Pharmacol. 2005, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Crassous, P.A.; Shu, P.; Huang, C.; Gordan, R.; Brouckaert, P.; Lampe, P.D.; Xie, L.H.; Beuve, A. Newly Identified NO-Sensor Guanylyl Cyclase/Connexin 43 Association Is Involved in Cardiac Electrical Function. J. Am. Heart Assoc. 2017, 6, e006397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, N.; Zhang, X.; Chen, D.; Li, Z. The Controversial Role of Adiponectin in Appetite Regulation of Animals. Nutrients 2021, 13, 3387. [Google Scholar] [CrossRef] [PubMed]
- Ziora-Jakutowicz, K.N.; Zimowski, J.; Ziora, K.; Bednarska-Makaruk, M.; Świętochowska, E.; Gorczyca, P.; Szczepańska, M.; Machura, E.; Stojewska, M.; Gołąb-Jenerał, K.; et al. Evaluation of the frequency of ADIPOQ c.45 T>G and ADIPOQ c.276 G>T polymorphisms in adiponectin coding gene in girls with anorexia nervosa. Endokrynol. Pol. 2021, 72, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Di Giorgio, M.R.; Rossi, E.; Tozzi, R.; Contini, S.; Bauleo, L.; Cipriani, F.; Toscano, R.; Basciani, S.; Barbaro, G.; et al. Blood SIRT1 Shows a Coherent Association with Leptin and Adiponectin in Relation to the Degree and Distribution of Adiposity: A Study in Obesity, Normal Weight and Anorexia Nervosa. Nutrients 2020, 12, 3506. [Google Scholar] [CrossRef]
- Sato, Y.; Fukudo, S. Gastrointestinal symptoms and disorders in patients with eating disorders. Clin. J. Gastroenterol. 2015, 8, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Massanet, P.L.; Petit, L.; Louart, B.; Corne, P.; Richard, C.; Preiser, J.C. Nutrition rehabilitation in the intensive care unit. JPEN. J. Parentel. Enteral. Nutr. 2015, 39, 391–400. [Google Scholar] [CrossRef]
- Feinle-Bisset, C. Upper gastrointestinal sensitivity to meal-related signals in adult humans-relevance to appetite regulation and gut symptoms in health, obesity and functional dyspepsia. Physiol. Behav. 2016, 162, 69–82. [Google Scholar] [CrossRef]
- Tagami, T.; Satoh, N.; Usui, T.; Yamada, K.; Shimatsu, A.; Kuzuya, H. Adiponectin in anorexia nervosa and bulimia nervosa. J. Clin. Endocrinol. Metab. 2004, 89, 1833–1837. [Google Scholar] [CrossRef]
- Schalla, M.A.; Stengel, A. Activity Based Anorexia as an Animal Model for Anorexia Nervosa-A Systematic Review. Front. Nutr. 2019, 6, 69. [Google Scholar] [CrossRef]
- Pucci, M.; D’Addario, C.; Micioni Di Bonaventura, E.; Mercante, F.; Annunzi, E.; Fanti, F.; Sergi, M.; Botticelli, L.; Einaudi, G.; Cifani, C.; et al. Endocannabinoid System Regulation in Female Rats with Recurrent Episodes of Binge Eating. Int. J. Mol. Sci. 2022, 23, 15228. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.F.; Liao, Y.; Hsueh, M.C.; Yen, H.Y.; Park, J.H.; Chang, J.H. Substituting sedentary time with physical activity in youngest-old to oldest-old community-dwelling older adults: Associations with body composition. Front. Public Health 2022, 10, 837213. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P. Why most published research findings are false. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sassoli, C.; Garella, R.; Chellini, F.; Tani, A.; Pavan, P.; Bambi, F.; Zecchi-Orlandini, S.; Squecco, R. Platelet-rich plasma affects gap junctional features in myofibroblasts in vitro via vascular endothelial growth factor (VEGF)-A/VEGF receptor. Exp. Physiol. 2022, 107, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Statistics Kingdom Home Page. Available online: https://www.statskingdom.com/shapiro-wilk-test-calculator.html (accessed on 31 July 2022).
- Jamovi Open Statistical Software Home Page. Available online: https://www.jamovi.org (accessed on 31 October 2022).
- The R Project for Statistical Computing Home Page. R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.R-project.org/. (accessed on 31 January 2022).





| CTRL | ADPN | ODQ | ODQ + ADPN | |
|---|---|---|---|---|
| RMP (mV) | −30.9 ± 3.3 | −44.0 ± 11.3 * | −29.1 ± 5.7 # | −28.5 ± 6.4 # |
| Cm (pF) | 17.0 ± 9.8 | 24.7 ± 4.2 * | 16.4 ± 8.6 | 15.8 ± 8.1 |
| AN (n = 34) | BN (n = 14) | BED (n = 14) | HC (n = 41) | F | |
|---|---|---|---|---|---|
| ADPN (µg/mL) | 11.95 ± 4.91 | 12.08 ± 3.30 | 13.30 ± 5.68 | 11.37 ± 4.80 | 0.51 |
| Age- and BMI-Adjusted β | |
|---|---|
| EDE-Q dietary restraint | −0.10 |
| EDE-Q eating concern | −0.08 |
| EDE-Q weight concern | −0.09 |
| EDE-Q shape concern | −0.09 |
| EDE-Q total score | −0.10 |
| Total overeating episodes | −0.01 |
| Objective binge-eating episodes | 0.08 |
| Subjective binge-eating episodes | −0.12 |
| Self-induced vomiting | 0.03 |
| Laxatives | −0.00 |
| Diuretics | 0.18 |
| Compensatory exercise episodes | 0.14 |
| EES anger | 0.05 |
| EES anxiety | 0.01 |
| EES depression | 0.03 |
| EES total score | 0.03 |
| SCL-90-R GSI | −0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garella, R.; Cassioli, E.; Chellini, F.; Tani, A.; Rossi, E.; Idrizaj, E.; Guasti, D.; Comeglio, P.; Palmieri, F.; Parigi, M.; et al. Defining the Molecular Mechanisms of the Relaxant Action of Adiponectin on Murine Gastric Fundus Smooth Muscle: Potential Translational Perspectives on Eating Disorder Management. Int. J. Mol. Sci. 2023, 24, 1082. https://doi.org/10.3390/ijms24021082
Garella R, Cassioli E, Chellini F, Tani A, Rossi E, Idrizaj E, Guasti D, Comeglio P, Palmieri F, Parigi M, et al. Defining the Molecular Mechanisms of the Relaxant Action of Adiponectin on Murine Gastric Fundus Smooth Muscle: Potential Translational Perspectives on Eating Disorder Management. International Journal of Molecular Sciences. 2023; 24(2):1082. https://doi.org/10.3390/ijms24021082
Chicago/Turabian StyleGarella, Rachele, Emanuele Cassioli, Flaminia Chellini, Alessia Tani, Eleonora Rossi, Eglantina Idrizaj, Daniele Guasti, Paolo Comeglio, Francesco Palmieri, Martina Parigi, and et al. 2023. "Defining the Molecular Mechanisms of the Relaxant Action of Adiponectin on Murine Gastric Fundus Smooth Muscle: Potential Translational Perspectives on Eating Disorder Management" International Journal of Molecular Sciences 24, no. 2: 1082. https://doi.org/10.3390/ijms24021082