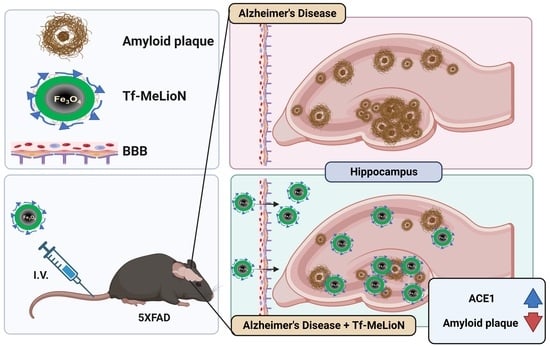
Transferrin-Conjugated Melittin-Loaded L-Arginine-Coated Iron Oxide Nanoparticles for Mitigating Beta-Amyloid Pathology of the 5XFAD Mouse Brain
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Characterization of Tf-MeLioNs
2.2. Biosafety Profile of Tf-MeLioNs In Vitro and In Vivo
2.3. Effects of Tf-MeLioNs Treatment on the Number of Amyloid Plaques in the 5XFAD Mice
2.4. Microglial Activation in the Hippocampal DG upon Tf-MeLioNs Treatment of 5XFAD Mice
2.5. Effect of Tf-MeLioNs on Proteins Related to Amyloid Regulation in the Hippocampus
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animal Experiments and Sampling
5.2. Synthesis of Tf-MeLioNs
5.3. Transmission and Scanning Electron Microscopy Imaging
5.4. FT-IR Spectroscopy
5.5. MALDI-TOF Spectrometry
5.6. Cell Culture (RAW 264.7 and C166)
5.7. Cytotoxicity Assay
5.8. Hemolytic Activity Assay
5.9. Drug Release Test
5.10. Tissue Hematoxylin–Eosin and Prussian Blue Staining
5.11. In Vivo Tf-MeLioNs Tracking
5.12. Thioflavin S Staining
5.13. Immunofluorescence Staining
5.14. Western Blot
5.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalaria, R.N.; Maestre, G.E.; Arizaga, R.; Friedland, R.P.; Galasko, D.; Hall, K.; Luchsinger, J.A.; Ogunniyi, A.; Perry, E.K.; Potocnik, F.; et al. Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol. 2008, 7, 812–826. [Google Scholar] [CrossRef] [PubMed]
- 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [CrossRef] [PubMed]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef]
- Crous-Bou, M.; Minguillon, C.; Gramunt, N.; Molinuevo, J.L. Alzheimer’s disease prevention: From risk factors to early intervention. Alzheimers Res. Ther. 2017, 9, 71. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Jantti, H.; Sitnikova, V.; Ishchenko, Y.; Shakirzyanova, A.; Giudice, L.; Ugidos, I.F.; Gomez-Budia, M.; Korvenlaita, N.; Ohtonen, S.; Belaya, I.; et al. Microglial amyloid beta clearance is driven by PIEZO1 channels. J. Neuroinflammation 2022, 19, 147. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.; Parbo, P.; Madsen, L.S.; Hansen, A.K.; Hansen, K.V.; Schaldemose, J.L.; Kjeldsen, P.L.; Stokholm, M.G.; Gottrup, H.; Eskildsen, S.F.; et al. The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: A longitudinal PET study. J. Neuroinflammation 2020, 17, 1–11. [Google Scholar] [CrossRef]
- Lin, T.Y.; Hsieh, C.L. Clinical Applications of Bee Venom Acupoint Injection. Toxins 2020, 12, 618. [Google Scholar] [CrossRef]
- Nguyen, C.D.; Lee, G. Neuroprotective Activity of Melittin-The Main Component of Bee Venom-Against Oxidative Stress Induced by Abeta(25–35) in In Vitro and In Vivo Models. Antioxidants 2021, 10, 1654. [Google Scholar] [CrossRef]
- Nguyen, C.D.; Yoo, J.; Hwang, S.Y.; Cho, S.Y.; Kim, M.; Jang, H.; No, K.O.; Shin, J.C.; Kim, J.H.; Lee, G. Bee Venom Activates the Nrf2/HO-1 and TrkB/CREB/BDNF Pathways in Neuronal Cell Responses against Oxidative Stress Induced by Abeta(1–42). Int. J. Mol. Sci. 2022, 23, 1193. [Google Scholar] [CrossRef]
- Yoo, J.; Lee, G. Adverse Events Associated with the Clinical Use of Bee Venom: A Review. Toxins 2022, 14, 562. [Google Scholar] [CrossRef]
- Jang, S.; Kim, K.H. Clinical Effectiveness and Adverse Events of Bee Venom Therapy: A Systematic Review of Randomized Controlled Trials. Toxins 2020, 12, 558. [Google Scholar] [CrossRef]
- Vu, H.D.; Huynh, P.T.; Ryu, J.; Kang, U.R.; Youn, S.W.; Kim, H.; Ahn, H.J.; Park, K.; Hwang, S.K.; Chang, Y.C.; et al. Melittin-loaded Iron Oxide Nanoparticles Prevent Intracranial Arterial Dolichoectasia Development through Inhibition of Macrophage-mediated Inflammation. Int. J. Biol. Sci. 2021, 17, 3818–3836. [Google Scholar] [CrossRef] [PubMed]
- Magro, M.; Baratella, D.; Bonaiuto, E.; de A Roger, J.; Vianello, F. New Perspectives on Biomedical Applications of Iron Oxide Nanoparticles. Curr. Med. Chem. 2018, 25, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Ajinkya, N.; Yu, X.; Kaithal, P.; Luo, H.; Somani, P.; Ramakrishna, S. Magnetic Iron Oxide Nanoparticle (IONP) Synthesis to Applications: Present and Future. Materials 2020, 13, 4644. [Google Scholar] [CrossRef]
- Wu, G.; Meininger, C.J.; McNeal, C.J.; Bazer, F.W.; Rhoads, J.M. Role of L-Arginine in Nitric Oxide Synthesis and Health in Humans. Adv. Exp. Med. Biol. 2021, 1332, 167–187. [Google Scholar]
- Pardridge, W.M. Treatment of Alzheimer’s Disease and Blood-Brain Barrier Drug Delivery. Pharmaceuticals 2020, 13, 394. [Google Scholar] [CrossRef]
- Zhou, L.; Kodidela, S.; Godse, S.; Thomas-Gooch, S.; Kumar, A.; Raji, B.; Zhi, K.; Kochat, H.; Kumar, S. Targeted Drug Delivery to the Central Nervous System Using Extracellular Vesicles. Pharmaceuticals 2022, 15, 358. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef]
- Moos, T.; Morgan, E.H. Transferrin and transferrin receptor function in brain barrier systems. Cell Mol. Neurobiol. 2000, 20, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Johnsen, K.B.; Kucharz, K.; Lauritzen, M.; Moos, T. Blood-Brain Barrier Transport of Transferrin Receptor-Targeted Nanoparticles. Pharmaceutics 2022, 14, 2237. [Google Scholar] [CrossRef]
- Shen, J.; Zhao, Z.; Shang, W.; Liu, C.; Zhang, B.; Xu, Z.; Cai, H. Fabrication and evaluation a transferrin receptor targeting nano-drug carrier for cerebral infarction treatment. Artif. Cells Nanomed. Biotechnol. 2019, 47, 192–200. [Google Scholar] [CrossRef]
- Rao, Y.L.; Ganaraja, B.; Murlimanju, B.V.; Joy, T.; Krishnamurthy, A.; Agrawal, A. Hippocampus and its involvement in Alzheimer’s disease: A review. 3 Biotech 2022, 12, 55. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, D.A.E.; van Eden, C.G.; Schuurman, K.G.; Hamann, J.; Huitinga, I. Staining of HLA-DR, Iba1 and CD68 in human microglia reveals partially overlapping expression depending on cellular morphology and pathology. J. Neuroimmunol. 2017, 309, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.E.; Ko, T.; Koh, H.S.; Ji, Y.W.; Shin, J.; Kim, K.; Kim, H.Y.; Lee, H.K.; Kim, Y. Alterations of aqueous humor Abeta levels in Abeta-infused and transgenic mouse models of Alzheimer disease. PLoS ONE 2020, 15, e0227618. [Google Scholar] [CrossRef] [PubMed]
- Robakis, N.K.; Efthimiopoulos, S. Familial Alzheimer disease: Changes in Abeta production may indicate a disturbance in protein transport or function caused by pleiotropic effects of FAD mutations. Neurobiol. Aging 1999, 20, 81–83, discussion 87. [Google Scholar]
- Liebsch, F.; Kulic, L.; Teunissen, C.; Shobo, A.; Ulku, I.; Engelschalt, V.; Hancock, M.A.; van der Flier, W.M.; Kunach, P.; Rosa-Neto, P.; et al. Abeta34 is a BACE1-derived degradation intermediate associated with amyloid clearance and Alzheimer’s disease progression. Nat. Commun. 2019, 10, 2240. [Google Scholar] [CrossRef]
- Guenette, S.Y. Mechanisms of Abeta clearance and catabolism. Neuromolecular Med. 2003, 4, 147–160. [Google Scholar] [CrossRef]
- Eckman, E.A.; Eckman, C.B. Abeta-degrading enzymes: Modulators of Alzheimer’s disease pathogenesis and targets for therapeutic intervention. Biochem. Soc. Trans. 2005, 33 Pt 5, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.F.; Elbert, D.L.; Kasten, T.P.; Patterson, B.W.; Sigurdson, W.C.; Connors, R.E.; Ovod, V.; Munsell, L.Y.; Mawuenyega, K.G.; Miller-Thomas, M.M.; et al. Amyloid-beta efflux from the central nervous system into the plasma. Ann. Neurol. 2014, 76, 837–844. [Google Scholar] [CrossRef]
- Deane, R.; Du Yan, S.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J.; et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003, 9, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Sevigny, J.; Chiao, P.; Bussiere, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. Addendum: The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature 2017, 546, 564. [Google Scholar] [CrossRef]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of Anti-amyloid-beta Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer’s Disease: A Focus on Aducanumab and Lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef] [PubMed]
- Tolar, M.; Abushakra, S.; Hey, J.A.; Porsteinsson, A.; Sabbagh, M. Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimers Res. Ther. 2020, 12, 95. [Google Scholar] [CrossRef]
- Zhang, C.; Wan, X.; Zheng, X.; Shao, X.; Liu, Q.; Zhang, Q.; Qian, Y. Dual-functional nanoparticles targeting amyloid plaques in the brains of Alzheimer’s disease mice. Biomaterials 2014, 35, 456–465. [Google Scholar] [CrossRef]
- Minett, T.; Classey, J.; Matthews, F.E.; Fahrenhold, M.; Taga, M.; Brayne, C.; Ince, P.G.; Nicoll, J.A.R.; Boche, D.; Cfas, M. Microglial immunophenotype in dementia with Alzheimer’s pathology. J. Neuroinflammation 2016, 13, 1–10. [Google Scholar] [CrossRef]
- Chen, F.; Yang, D.; Cheng, X.Y.; Yang, H.; Yang, X.H.; Liu, H.T.; Wang, R.; Zheng, P.; Yao, Y.; Li, J. Astragaloside IV Ameliorates Cognitive Impairment and Neuroinflammation in an Oligomeric A beta Induced Alzheimer’s Disease Mouse Model via Inhibition of Microglial Activation and NADPH Oxidase Expression. Biol. Pharm. Bull. 2021, 44, 1688–1696. [Google Scholar] [CrossRef]
- Hopperton, K.; Mohammad, D.; Trepanier, M.; Giuliano, V.; Bazinet, R.P. Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: A systematic review. Mol. Psychiatry 2018, 23, 177–198. [Google Scholar] [CrossRef]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Zhang, L.L.; Liu, Y.Z.; Wang, X.; Wang, D.; Wu, H.; Chen, H.C.; Chen, J.X.; Liu, Y.P. Treadmill exercise improve recognition memory by TREM2 pathway to inhibit hippocampal microglial activation and neuroinflammation in Alzheimer’s disease model. Physiol. Behav. 2022, 251, 113820. [Google Scholar] [CrossRef]
- Long, H.Z.; Zhou, Z.W.; Cheng, Y.; Luo, H.Y.; Li, F.J.; Xu, S.G.; Gao, L.C. The Role of Microglia in Alzheimer’s Disease From the Perspective of Immune Inflammation and Iron Metabolism. Front. Aging Neurosci. 2022, 14, 888989. [Google Scholar] [CrossRef]
- Lian, H.; Litvinchuk, A.; Chiang, A.C.A.; Aithmitti, N.; Jankowsky, J.L.; Zheng, H. Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer’s Disease. J. Neurosci. 2016, 36, 577–589. [Google Scholar] [CrossRef]
- Frautschy, S.A.; Yang, F.S.; Irrizarry, M.; Hyman, B.; Saido, T.C.; Hsiao, K.; Cole, G.M. Microglial response to amyloid plaques in APPsw transgenic mice. Am. J. Pathol. 1998, 152, 307–317. [Google Scholar] [PubMed]
- Okello, A.; Edison, P.; Archer, H.A.; Turkheimer, F.E.; Kennedy, J.; Bullock, R.; Walker, Z.; Kennedy, A.; Fox, N.; Rossor, M.; et al. Microglial activation and amyloid deposition in mild cognitive impairment A PET study. Neurology 2009, 72, 56–62. [Google Scholar] [CrossRef]
- Miao, J.F.; Ma, H.X.; Yang, Y.; Liao, Y.P.; Lin, C.; Zheng, J.X.; Yu, M.L.; Lan, J. Microglia in Alzheimer’s disease: Pathogenesis, mechanisms, and therapeutic potentials. Front. Aging Neurosci. 2023, 15, 565–577. [Google Scholar] [CrossRef]
- Fu, W.Y.; Wang, X.J.; Ip, N.Y. Targeting Neuroinflammation as a Therapeutic Strategy for Alzheimer’s Disease: Mechanisms, Drug Candidates, and New Opportunities. ACS Chem. Neurosci. 2019, 10, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H. Alzheimer’s Disease and the Amyloid-beta Peptide. J. Alzheimers Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Bell, R.D.; Sagare, A.; Zlokovic, B.V. Clearance of Amyloid-beta Peptide Across the Blood-Brain Barrier: Implication for Therapies in Alzheimer’s Disease. CNS Neurol. Disord.-Drug Targets 2009, 8, 16–30. [Google Scholar] [CrossRef]
- Saido, T.; Leissring, M.A. Proteolytic Degradation of Amyloid beta-Protein. CSH Perspect. Med. 2012, 2, a006379. [Google Scholar]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Toropygin, I.Y.; Kugaevskaya, E.V.; Mirgorodskaya, O.A.; Elisseeva, Y.E.; Kozmin, Y.P.; Popov, I.A.; Nikolaev, E.N.; Makarov, A.A.; Kozin, S.A. The N-domain of angiotensin-converting enzyme specifically hydrolyzes the Arg-5-His-6 bond of Alzheimer’s Abeta-(1–16) peptide and its isoAsp-7 analogue with different efficiency as evidenced by quantitative matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 231–239. [Google Scholar] [PubMed]
- Ambrocio-Ortiz, E.; Perez-Rubio, G.; Del Angel-Pablo, A.D.; Buendia-Roldan, I.; Chavez-Galan, L.; Hernandez-Zenteno, R.J.; Ramirez-Venegas, A.; Rojas-Serrano, J.; Mejia, M.; Perez-Padilla, R.; et al. Angiotensin-Converting Enzyme 2 (ACE2) in the Context of Respiratory Diseases and Its Importance in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Pharmaceuticals 2021, 14, 805. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Saito, Y. Roles of Natriuretic Peptides and the Significance of Neprilysin in Cardiovascular Diseases. Biology 2022, 11, 1017. [Google Scholar] [CrossRef]
- Chesneau, V.; Vekrellis, K.; Rosner, M.R.; Selkoe, D.J. Purified recombinant insulin-degrading enzyme degrades amyloid beta-protein but does not promote its oligomerization. Biochem. J. 2000, 351 Pt 2, 509–516. [Google Scholar] [CrossRef]
- Hernandez-Guillamon, M.; Mawhirt, S.; Blais, S.; Montaner, J.; Neubert, T.A.; Rostagno, A.; Ghiso, J. Sequential Amyloid-beta Degradation by the Matrix Metalloproteases MMP-2 and MMP-9. J. Biol. Chem. 2015, 290, 15078–15091. [Google Scholar] [CrossRef]
- Jochemsen, H.M.; Teunissen, C.E.; Ashby, E.L.; van der Flier, W.M.; Jones, R.E.; Geerlings, M.I.; Scheltens, P.; Kehoe, P.G.; Muller, M. The association of angiotensin-converting enzyme with biomarkers for Alzheimer’s disease. Alzheimers Res. Ther. 2014, 6, 27. [Google Scholar] [CrossRef]
- Cortes-Canteli, M.; Iadecola, C. Alzheimer’s Disease and Vascular Aging: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 942–951. [Google Scholar] [CrossRef]
- Faraco, G.; Park, L.; Zhou, P.; Luo, W.; Paul, S.M.; Anrather, J.; Iadecola, C. Hypertension enhances Abeta-induced neurovascular dysfunction, promotes beta-secretase activity, and leads to amyloidogenic processing of APP. J. Cereb. Blood Flow Metab. 2016, 36, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chang, X.; Lang, M. Iron Homeostasis Disorder and Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 12442. [Google Scholar] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Valensin, D.; Gabbiani, C.; Messori, L. Metal compounds as inhibitors of beta-amyloid aggregation. Perspectives for an innovative metallotherapeutics on Alzheimer’s disease. Coordin. Chem. Rev. 2012, 256, 2357–2366. [Google Scholar] [CrossRef]
- Strodel, B.; Coskuner-Weber, O. Transition Metal Ion Interactions with Disordered Amyloid-beta Peptides in the Pathogenesis of Alzheimer’s Disease: Insights from Computational Chemistry Studies. J. Chem. Inf. Model 2019, 59, 1782–1805. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Fan, Y.G.; Yang, Z.S.; Wang, Z.Y.; Guo, C. Iron and Alzheimer’s Disease: From Pathogenesis to Therapeutic Implications. Front. Neurosci. 2018, 12, 632. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, J.; Fujita, Y.; Liu, S.; Maeda, T.; Kikuchi, K.; Obara, T.; Takebe, A.; Sayama, R.; Takahashi, T.; et al. Iron treatment inhibits Abeta42 deposition in vivo and reduces Abeta42/Abeta40 ratio. Biochem. Biophys. Res. Commun. 2019, 512, 653–658. [Google Scholar] [CrossRef]
- Choi, M.; Kim, D.; Youn, Y.-J.; Ryu, J.; Jeong, Y.H. Effect of Obesity and High-Density Lipoprotein Concentration on the Pathological Characteristics of Alzheimer’s Disease in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2022, 23, 12296. [Google Scholar] [CrossRef]
- Choi, M.; Lee, S.M.; Kim, D.; Im, H.I.; Kim, H.S.; Ha Jeong, Y. Disruption of the astrocyte-neuron interaction is responsible for the impairments in learning and memory in 5XFAD mice: An Alzheimer’s disease animal model. Mol. Brain 2021, 14, 1–5. [Google Scholar] [CrossRef]
- Choi, M.; Kim, H.; Yang, E.J.; Kim, H.S. Inhibition of STAT3 phosphorylation attenuates impairments in learning and memory in 5XFAD mice, an animal model of Alzheimer’s disease. J. Pharmacol. Sci. 2020, 143, 290–299. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.; Ryu, J.; Vu, H.D.; Kim, D.; Youn, Y.-J.; Park, M.H.; Huynh, P.T.; Hwang, G.-B.; Youn, S.W.; Jeong, Y.H. Transferrin-Conjugated Melittin-Loaded L-Arginine-Coated Iron Oxide Nanoparticles for Mitigating Beta-Amyloid Pathology of the 5XFAD Mouse Brain. Int. J. Mol. Sci. 2023, 24, 14954. https://doi.org/10.3390/ijms241914954
Choi M, Ryu J, Vu HD, Kim D, Youn Y-J, Park MH, Huynh PT, Hwang G-B, Youn SW, Jeong YH. Transferrin-Conjugated Melittin-Loaded L-Arginine-Coated Iron Oxide Nanoparticles for Mitigating Beta-Amyloid Pathology of the 5XFAD Mouse Brain. International Journal of Molecular Sciences. 2023; 24(19):14954. https://doi.org/10.3390/ijms241914954
Chicago/Turabian StyleChoi, Moonseok, Junghwa Ryu, Huy Duc Vu, Dongsoo Kim, Young-Jin Youn, Min Hui Park, Phuong Tu Huynh, Gyu-Bin Hwang, Sung Won Youn, and Yun Ha Jeong. 2023. "Transferrin-Conjugated Melittin-Loaded L-Arginine-Coated Iron Oxide Nanoparticles for Mitigating Beta-Amyloid Pathology of the 5XFAD Mouse Brain" International Journal of Molecular Sciences 24, no. 19: 14954. https://doi.org/10.3390/ijms241914954