LQFM289: Electrochemical and Computational Studies of a New Trimetozine Analogue for Anxiety Treatment
Abstract
:1. Introduction
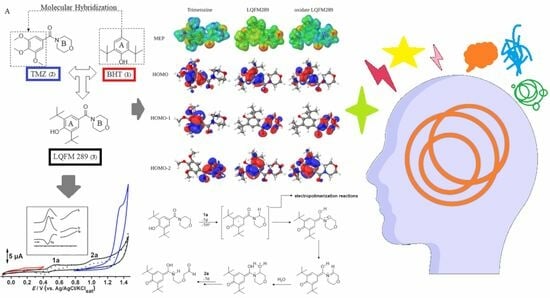
2. Results
2.1. Electrochemical Characterization of LQFM289 (3) Lead Compound
2.2. Quantitative Applications
2.3. Structural Characterization of LQFM289 (3)
3. Discussion
3.1. Structural Characterization of LQFM289 (3)
3.2. Computational Data and Electrochemical Characterization
3.3. Proposed Electrooxidation Mechanism
3.4. Quantitative Applications
4. Materials and Methods
4.1. Reagents, Samples and Solutions
4.2. Electrochemical Assays
4.3. Computational Studies
4.4. Crystal Structure Determinations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Rodrigues, E.S.B.; de Macêdo, I.Y.L.; da Silva Lima, L.L.; Thomaz, D.V.; da Cunha, C.E.P.; Teles de Oliveira, M.; Ballaminut, N.; Alecrim, M.F.; Ferreira de Carvalho, M.; Isecke, B.G.; et al. Electrochemical Characterization of Central Action Tricyclic Drugs by Voltammetric Techniques and Density Functional Theory Calculations. Pharmaceuticals 2019, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Sena, T. Manual Diagnóstico e Estatístico de Transtornos Mentais—DSM-5, estatísticas e ciências humanas: Inflexões sobre normalizações e normatizações. Rev. Int. Interdiscip. Interthesis 2014, 11, 96. [Google Scholar] [CrossRef]
- Barlow, D.H. Clinical Handbook of Psychological Disorders, Fifth Edition: A Step-By-Step Treatment Manual; Guilford Publications: New York, NY, USA, 2014. [Google Scholar]
- Santana, I.G.C.; Almeida, L.D.S.; Moreira, L.K.D.S.; de Carvalho, F.S.; Menegatti, R.; da Rocha, A.L.B.; Mazurok, T.A.; Vaz, B.G.; Lião, L.M.; Brito, A.F.; et al. Structure-activity relationship of three new piperazine derivates with anxiolytic-like and antidepressant-like effects. Can. J. Physiol. Pharmacol. 2022, 100, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Tiller, J.W.G. Depression and anxiety. Med. J. Aust. 2013, 199, S28–S31. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Rammal, H.; Soulimani, R. Oxidative Stress and Anxiety: Relationship and Cellular Pathways. Oxidative Med. Cell. Longev. 2009, 2, 63–67. [Google Scholar] [CrossRef]
- Fedoce, A.D.G.; Ferreira, F.; Bota, R.G.; Bonet-Costa, V.; Sun, P.Y.; Davies, K.J.A. The role of oxidative stress in anxiety disorder: Cause or consequence? Free Radic. Res. 2018, 52, 737–750. [Google Scholar] [CrossRef]
- Neurauter, G.; Schröcksnadel, K.; Scholl-Bürgi, S.; Sperner-Unterweger, B.; Schubert, C.; Ledochowski, M.; Fuchs, D. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr. Drug Metab. 2008, 9, 622–627. [Google Scholar] [CrossRef]
- Moreira, L.K.S.; Turones, L.C.; Campos, H.M.; Nazaré, A.M.; Thomaz, D.V.; Gil, E.S.; Ghedini, P.C.; Rocha, F.F.; Menegatti, R.; Fajemiroye, J.O.; et al. LQFM212, a piperazine derivative, exhibits potential antioxidant effect as well as ameliorates LPS-induced behavioral, inflammatory and oxidative changes. Life Sci. 2023, 312, 121199. [Google Scholar] [CrossRef]
- Moigneu, C.; Abdellaoui, S.; Ramos-Brossier, M.; Pfaffenseller, B.; Wollenhaupt-Aguiar, B.; de Azevedo Cardoso, T.; Chiche, A.; Kuperwasser, N.; Azevedo da Silva, R.; Pedrotti Moreira, F.; et al. Systemic GDF11 attenuates depression-like phenotype in aged mice via stimulation of neuronal autophagy. Nat. Aging 2023, 3, 213–228. [Google Scholar] [CrossRef]
- Jadon, N.; Jain, R.; Aribam, N.G.; Chauhan, P. Review—Monitoring of Endogenous Antioxidants: An Electroanalytical Approach. J. Electrochem. Soc. 2017, 164, H266–H277. [Google Scholar] [CrossRef]
- Conti, V.; Izzo, V.; Corbi, G.; Russomanno, G.; Manzo, V.; De Lise, F.; Di Donato, A.; Filippelli, A. Antioxidant Supplementation in the Treatment of Aging-Associated Diseases. Front. Pharmacol. 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, M.; Tezcan, E.; Kuloglu, M.; Ustundag, B.; Tunckol, H. Antioxidant enzyme and malondialdehyde values in social phobia before and after citalopram treatment. Eur. Arch. Psychiatry Clin. Neurosci. 2004, 254, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Girish, C.; Raj, V.; Arya, J.; Balakrishnan, S. Involvement of the GABAergic system in the anxiolytic-like effect of the flavonoid ellagic acid in mice. Eur. J. Pharmacol. 2013, 710, 49–58. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Li, H.; Tang, Y.; Yang, L.; Cao, S.; Qin, D. Antidepressant Potential of Chlorogenic Acid-Enriched Extract from Eucommia ulmoides Oliver Bark with Neuron Protection and Promotion of Serotonin Release through Enhancing Synapsin I Expression. Molecules 2016, 21, 260. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.A.; Moreno, D.H.; Soares, M.B.D.M. Psicofarmacologia de antidepressivos. Rev. Bras. Psiquiatr. 1999, 21 (Suppl. S1), 24–40. [Google Scholar] [CrossRef]
- Araújo, C.R.M.; Leite Filho, C.A.; Santos, V.L.D.A.; Maia, G.L.D.A.; Gonsalves, A.D.A. Drug Development by Molecular Hybridization: A Medicinal Chemistry Practice Class Using Paracetamol and Sulfadiazine Tablets and the Virtual Tool SciFinder®. Quim. Nova 2015, 38, 868–873. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 12478, Trimetozine. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Trimetozine (accessed on 1 April 2023).
- Cascade, E.; Kalali, H.; Kennedy, H. Real-World Data on SSRI Antidepressant Side Effects. Psychiatry 2009, 6, 16–18. [Google Scholar]
- Kourounakis, A.P.; Xanthopoulos, D.; Tzara, A. Morpholine as a privileged structure: A review on the medicinal chemistry and pharmacological activity of morpholine containing bioactive molecules. Med. Res. Rev. 2020, 40, 709–752. [Google Scholar] [CrossRef]
- Rekka, E.A.; Kourounakis, P.N. Medicinal Chemistry of 2,2,4-Substituted Morpholines. Curr. Med. Chem. 2010, 17, 3422–3430. [Google Scholar] [CrossRef]
- Obiwulu, E. Protective function of butylated hydroxytoluene in lead-induced oxidative alterations in tissues of Wistar rats. J. Appl. Sci. Environ. Manag. 2020, 23, 2051. [Google Scholar] [CrossRef]
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): A review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Celestino, M.T.; Magalhães, U.D.O.; Fraga, A.G.M.; Carmo, F.A.D.; Lione, V.; Castro, H.C.; Sousa, V.P.D.; Rodrigues, C.R.; Cabral, L.M. Rational use of antioxidants in solid oral pharmaceutical preparations. Braz. J. Pharm. Sci. 2012, 48, 405–415. [Google Scholar] [CrossRef]
- Brito, A.F.; Braga, P.C.C.S.; Moreira, L.K.S.; Silva, D.M.; Silva, D.P.B.; Sanz, G.; Vaz, B.G.; de Carvalho, F.S.; Lião, L.M.; Silva, R.R.; et al. A new piperazine derivative: 1-(4-(3,5-di-tert-butyl-4-hydroxybenzyl) piperazin-1-yl)-2-methoxyethan-1-one with antioxidant and central activity. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 391, 255–269. [Google Scholar] [CrossRef]
- Cabral, I.B.; Moreira, C.V.; Rodrigues, A.C.; Moreira, L.K.; Pereira, J.K.; Gomides, C.D.; Lião, L.M.; Machado, L.S.; Vaz, B.G.; Cunha, L.C.; et al. Preclinical data on morpholine (3,5-di-tertbutyl-4-hydroxyphenyl) methanone induced anxiolysis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023. submitted. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Filho, D.; De Souza, D. Detection of Synthetic Antioxidants: What Factors Affect the Efficiency in the Chromatographic Analysis and in the Electrochemical Analysis? Molecules 2022, 27, 7137. [Google Scholar] [CrossRef]
- de Macêdo, I.Y.L.; Garcia, L.F.; Menegatti, R.; Guimarães, F.F.; Lião, L.M.; de Carvalho, F.S.; Torres Pio dos Santos, W.; Verly, R.M.; Arotiba, O.A.; de Souza Gil, E. Electrochemical characterizations of darbufelone, a di-tert-butylphenol derivative, by voltammetric techniques and density functional theory calculations. Electrochim. Acta 2018, 268, 462–468. [Google Scholar] [CrossRef]
- Gil, E.S.; Couto, R.O. Flavonoid Electrochemistry, a review on the electroanalytical applications. Braz. J. Pharmacogn. 2013, 23, 542–558. [Google Scholar] [CrossRef]
- Simić, A.; Manojlović, D.; Šegan, D.; Todorović, M. Electrochemical Behavior and Antioxidant and Prooxidant Activity of Natural Phenolics. Molecules 2007, 12, 2327–2340. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Sahnoun, R.; Broclawik, E.; Koyama, M.; Tsuboi, H.; Hatakeyama, N.; Endou, A.; Takaba, H.; Kubo, M.; Del Carpio, C.A.; et al. Quantum chemical studies for oxidation of morpholine by Cytochrome P450. J. Inorg. Biochem. 2009, 103, 20–27. [Google Scholar] [CrossRef]
- Mruthunjaya, A.K.V.; Torriero, A.A.J. Mechanistic Aspects of the Electrochemical Oxidation of Aliphatic Amines and Aniline Derivatives. Molecules 2023, 28, 471. [Google Scholar] [CrossRef]
- Roushani, M.; Sarabaegi, M. Electrochemical detection of butylated hydroxyanisole based on glassy carbon electrode modified by iridium oxide nanoparticles. J. Electroanal. Chem. 2014, 717–718, 147–152. [Google Scholar] [CrossRef]
- de Oliveira, S.M.; dos Santos Castro Assis, K.L.; Paiva, V.M.; Hashempour, M.; Bestetti, M.; de Araújo, J.R.; D’Elia, E. Facile electrochemical detection of morpholine in boiler water with carbon nanostructures: A comparative study of graphene and carbon nanotubes. Bull. Mater. Sci. 2022, 45, 100. [Google Scholar] [CrossRef]
- Combourieu, B.; Besse, P.; Sancelme, M.; Godin, J.-P.; Monteil, A.; Veschambre, H.; Delort, A.-M. Common Degradative Pathways of Morpholine, Thiomorpholine, and Piperidine by Mycobacterium aurum MO1: Evidence from1H-Nuclear Magnetic Resonance and Ionspray Mass Spectrometry Performed Directly on the Incubation Medium. Appl. Environ. Microbiol. 2000, 66, 3187–3193. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2018, 120, 215–241. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Schaftenaar, G. Molden 5.9.2. Available online: https://www3.cmbi.umcn.nl/molden/ (accessed on 1 July 2023).
- JMOL Development Team, JMOL. Available online: http://jmol.sourceforge.net/ (accessed on 7 October 2016).
- Suresh, C.H.; Remya, G.S.; Anjalikrishna, P.K. Molecular electrostatic potential analysis: A powerful tool to interpret and predict chemical reactivity. WIREs Comput. Mol. Sci. 2022, 12, e1601. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Louis, J. Farrugia, ORTEP-3 for Windows—A version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Louis, J. Farrugia, WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]








| Compound | (2) | (3) |
|---|---|---|
| Empirical formula | C14 H19 N O5 | C19 H29 N O3 |
| Formula weight | 281.30 | 319.43 |
| Crystal system | Triclinic | Monoclinic |
| Space group | P | P 21/n |
| a (Å) | 8.6525 (4) | 17.3332 (14) |
| b (Å) | 9.5424 (4) | 12.2252 (9) |
| c (Å) | 9.8544 (4) | 18.9650 (15) |
| α (°) | 64.2340 (10) | 90 |
| β (°) | 77.059 (2) | 110.427 (3) |
| γ (°) | 71.725 (2) | 90 |
| V (Å3) | 3692.12 (5) | 3766.0 (5) |
| Z | 2 | 8 |
| ρ (Mg/m3) | 1.350 | 1.127 |
| µ (mm−1) | 0.103 | 0.075 |
| Absorption correction | multi-scan | multi-scan |
| Reflections collected | 38,484 | 75,266 |
| Unique reflections/R(int) | 3918/0.0463 | 6655/0.1073 |
| Completeness to θ = 25.0° | 100.0% | 100.0% |
| Data/restraints/parameters | 3918/0/184 | 6655/0/430 |
| Goodness-of-fit | 1.130 | 1.084 |
| Final R1/wR2 [I > 2 σ(I)] | 0.0497/0.1466 | 0.1788/0.4337 |
| R1/wR2 indices (all data) | 0.0618/0.1529 | 0.2087/0.4521 |
| Extinction coefficient | -- | 0.0041 (15) |
| Largest diff. peak and hole (e.Å−3) | 0.359 and −0.226 | 0.880 and −0.449 |
| LQFM289-A | LQFM289-B | TMZ | TUJTAV | 289A-OPT | |
|---|---|---|---|---|---|
| O1-C1-C2-C7 | 36 (1) | 50 (1) | 46.6 (2) | 46.4 (2) | 37.61 |
| O1-C1-N1-C8 | 6 (1) | 8 (1) | 5.4 (2) | 5.8 (2) | 2.91 |
| O1-C1-N1-C10 | −154.0 (8) | −170.7 (9) | −158.1 (1) | −158.1 (1) | −149.03 |
| C2-C1-N1-C10 | 30 (1) | 13 (1) | 27.3 (2) | 26.8 (2) | 34.15 |
| C1-N1-C8-C10 | −162 (1) | −179 (1) | −165.3 (2) | −165.7 (2) | −155.25 |
| N1-C8-C9-O3 | 55 (1) | −56 (1) | −56.7 (2) | −55.7 (2) | 55.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.K.A.; Costa, A.G.C.; Rodrigues, E.S.B.; Macêdo, I.Y.L.; Pereira, M.O.A.; Menegatti, R.; de Oliveira, S.C.B.; Guimarães, F.; Lião, L.M.; Sabino, J.R.; et al. LQFM289: Electrochemical and Computational Studies of a New Trimetozine Analogue for Anxiety Treatment. Int. J. Mol. Sci. 2023, 24, 14575. https://doi.org/10.3390/ijms241914575
Pereira JKA, Costa AGC, Rodrigues ESB, Macêdo IYL, Pereira MOA, Menegatti R, de Oliveira SCB, Guimarães F, Lião LM, Sabino JR, et al. LQFM289: Electrochemical and Computational Studies of a New Trimetozine Analogue for Anxiety Treatment. International Journal of Molecular Sciences. 2023; 24(19):14575. https://doi.org/10.3390/ijms241914575
Chicago/Turabian StylePereira, Jhon K. A., André G. C. Costa, Edson S. B. Rodrigues, Isaac Y. L. Macêdo, Marx O. A. Pereira, Ricardo Menegatti, Severino C. B. de Oliveira, Freddy Guimarães, Luciano M. Lião, José R. Sabino, and et al. 2023. "LQFM289: Electrochemical and Computational Studies of a New Trimetozine Analogue for Anxiety Treatment" International Journal of Molecular Sciences 24, no. 19: 14575. https://doi.org/10.3390/ijms241914575