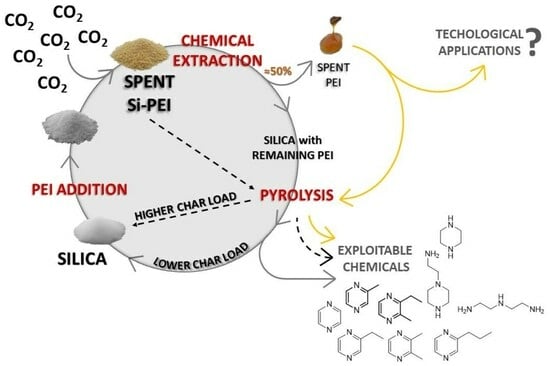
Valorization Strategies in CO2 Capture: A New Life for Exhausted Silica-Polyethylenimine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Si-PEI

2.2. Chemical Extraction of PEI from Si-PEI
2.2.1. TGA
2.2.2. Rheology
2.2.3. ATR-FTIR
2.2.4. 13C-AND 1H-NMR
2.2.5. Py-GC-MS
3. Materials and Methods
- SP0: fresh Si-PEI, 42.99 ± 0.06 wt% loading dried base.
- SP2: spent Si-PEI 42.5 ± 0.2 wt% loading 2%wt CO2.
- SP3: spent Si-PEI 42.8 ± 0.2 wt% loading 3%wt CO2.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Solomon, S.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible Climate Change Due to Carbon Dioxide Emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.D.; Weaver, A.J. Committed Climate Warming. Nat. Geosci. 2010, 3, 142–143. [Google Scholar] [CrossRef]
- Seneviratne, S.I.; Donat, M.G.; Pitman, A.J.; Knutti, R.; Wilby, R.L. Allowable CO2 Emissions Based on Regional and Impact-Related Climate Targets. Nature 2016, 529, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghussain, L. Global Warming: Review on Driving Forces and Mitigation: Global Warming: Review on Driving Forces and Mitigation. Environ. Prog. Sustain. Energy 2019, 38, 13–21. [Google Scholar] [CrossRef]
- Yoro, K.O.; Daramola, M.O. CO2 Emission Sources, Greenhouse Gases, and the Global Warming Effect. In Advances in Carbon Capture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–28. ISBN 978-0-12-819657-1. [Google Scholar]
- Markewitz, P.; Kuckshinrichs, W.; Leitner, W.; Linssen, J.; Zapp, P.; Bongartz, R.; Schreiber, A.; Müller, T.E. Worldwide Innovations in the Development of Carbon Capture Technologies and the Utilization of CO2. Energy Environ. Sci. 2012, 5, 7281. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An Overview of Current Status of Carbon Dioxide Capture and Storage Technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. The Role of Carbon Capture and Utilization, Carbon Capture and Storage, and Biomass to Enable a Net-Zero-CO2 Emissions Chemical Industry. Ind. Eng. Chem. Res. 2020, 59, 7033–7045. [Google Scholar] [CrossRef]
- Dziejarski, B.; Krzyżyńska, R.; Andersson, K. Current Status of Carbon Capture, Utilization, and Storage Technologies in the Global Economy: A Survey of Technical Assessment. Fuel 2023, 342, 127776. [Google Scholar] [CrossRef]
- Osman, A.I.; Hefny, M.; Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Rooney, D.W. Recent Advances in Carbon Capture Storage and Utilisation Technologies: A Review. Environ. Chem. Lett. 2021, 19, 797–849. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Bingre, R.; Huang, L.; Lutzweiler, G.; Wang, Q.; Louis, B. CO2 Adsorption Capacities in Zeolites and Layered Double Hydroxide Materials. Front. Chem. 2019, 7, 551. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y.; Serna-Guerrero, R. Flue Gas Treatment via CO2 Adsorption. Chem. Eng. J. 2011, 171, 760–774. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.-S.; Fisher, E.P.; Losch, J. Adsorption of CO2 on Zeolites at Moderate Temperatures. Energy Fuels 2005, 19, 1153–1159. [Google Scholar] [CrossRef]
- Su, H.; Yan, Y.; Zhang, J.-N.; Yan, W. CO2 Captured by Silicoaluminophosphate (SAPO) Zeotypes. Sustain. Chem. Clim. Action. 2023, 2, 100022. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. Chem.-Sustain.-Energy-Mater. 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Okolie, J.A.; Abdelrasoul, A.; Niu, C.; Dalai, A.K. Review of Post-Combustion Carbon Dioxide Capture Technologies Using Activated Carbon. J. Environ. Sci. 2019, 83, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon Capture and Conversion Using Metal–Organic Frameworks and MOF-Based Materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef]
- Yu, J.; Wang, P.; Ni, F.; Cizdziel, J.; Wu, D.; Zhao, Q.; Zhou, Y. Characterization of Microplastics in Environment by Thermal Gravimetric Analysis Coupled with Fourier Transform Infrared Spectroscopy. Mar. Pollut. Bull. 2019, 145, 153–160. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y. Stabilization of Amine-Containing CO2 Adsorbents: Dramatic Effect of Water Vapor. J. Am. Chem. Soc. 2010, 132, 6312–6314. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; De Weireld, G.; Sayari, A. Amine-Bearing Mesoporous Silica for CO 2 and H 2 S Removal from Natural Gas and Biogas. Langmuir 2009, 25, 13275–13278. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Serna-Guerrero, R.; Sayari, A. Adsorption of CO2 -Containing Gas Mixtures over Amine-Bbearing Pore-Expanded MCM-41 Silica: Application for Gas Purification. Ind. Eng. Chem. Res. 2010, 49, 359–365. [Google Scholar] [CrossRef]
- Didas, S.A.; Choi, S.; Chaikittisilp, W.; Jones, C.W. Amine–Oxide Hybrid Materials for CO 2 Capture from Ambient Air. Acc. Chem. Res. 2015, 48, 2680–2687. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.G.; Pevida, C.; Arias, B.; Casal, M.D.; Martín, C.F.; Fermoso, J.; Rubiera, F.; Pis, J.J. Different Approaches for the Development of Low-Cost CO2 Adsorbents. J. Environ. Eng. 2009, 135, 426–432. [Google Scholar] [CrossRef]
- Couck, S.; Denayer, J.F.M.; Baron, G.V.; Rémy, T.; Gascon, J.; Kapteijn, F. An Amine-Functionalized MIL-53 Metal−organic Framework WithlLarge Separation Power for CO2 and CH4. J. Am. Chem. Soc. 2009, 131, 6326–6327. [Google Scholar] [CrossRef] [PubMed]
- Vaidhyanathan, R.; Iremonger, S.S.; Dawson, K.W.; Shimizu, G.K.H. An Amine-Functionalized Metal Organic Framework for Preferential CO2 Adsorption at Low Pressures. Chem. Commun. 2009, 5230–5232. [Google Scholar] [CrossRef] [PubMed]
- Drage, T.C.; Arenillas, A.; Smith, K.M.; Snape, C.E. Thermal Stability of Polyethylenimine Based Carbon Dioxide Adsorbents and Its Influence on Selection of Regeneration Strategies. Microporous Mesoporous Mater. 2008, 116, 504–512. [Google Scholar] [CrossRef]
- Hahn, M.W.; Steib, M.; Jentys, A.; Lercher, J.A. Mechanism and Kinetics of CO2 Adsorption on Surface Bonded Amines. J. Phys. Chem. C 2015, 119, 4126–4135. [Google Scholar] [CrossRef]
- Sayari, A.; Heydari-Gorji, A.; Yang, Y. CO2-Induced Degradation of Amine-Containing Adsorbents: Reaction Products and Pathways. J. Am. Chem. Soc. 2012, 134, 13834–13842. [Google Scholar] [CrossRef]
- Serna-Guerrero, R.; Sayari, A. Applications of Pore-Expanded Mesoporous Silica. 7. Adsorption of Volatile Organic Compounds. Environ. Sci. Technol. 2007, 41, 4761–4766. [Google Scholar] [CrossRef]
- Wang, X.; Song, C. Carbon Capture from Flue Gas and the Atmosphere: A Perspective. Front. Energy Res. 2020, 8, 560849. [Google Scholar] [CrossRef]
- Akinola, T.E.; Bonilla Prado, P.L.; Wang, M. Experimental Studies, Molecular Simulation and Process Modelling\simulation of Adsorption-Based Post-Combustion Carbon Capture for Power Plants: A State-of-the-Art Review. Appl. Energy 2022, 317, 119156. [Google Scholar] [CrossRef]
- Zou, L.; Sun, Y.; Che, S.; Yang, X.; Wang, X.; Bosch, M.; Wang, Q.; Li, H.; Smith, M.; Yuan, S.; et al. Porous Organic Polymers for Post-combustion Carbon Capture. Adv. Mater. 2017, 29, 1700229. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Solid Waste Issue: Sources, Composition, Disposal, Recycling, and Valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical Routes for the Valorization of Waste Polyolefinic Plastics to Produce Fuels and Chemicals. A Review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.F.; Roelands, M.C.P.; White, R.J.; Harmelen, T.; Wild, P.; Laan, G.P.; Meirer, F.; Keurentjes, J.T.F.; Weckhuysen, B.M. Beyond Mechanical Recycling: Giving New Life to Plastic Waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, D.; Li, Y.C.; Wang, S.R. Biomass Valorization: Sustainable Methods for the Production of Chemicals. In Biomass Valorization. Sustainable Methods for the Production of Chemicals; Ravelli, D., Samorì, C., Eds.; Wiley-VCH: Weinheim, Germany, 2021; Chapter 6; pp. 149–180. ISBN 978-3-527-34717-9. [Google Scholar]
- Ahmadalinezhad, A.; Sayari, A. Oxidative Degradation of Silica-Supported Polyethylenimine for CO 2 Adsorption: Insights into the Nature of Deactivated Species. Phys. Chem. Chem. Phys. 2014, 16, 1529–1535. [Google Scholar] [CrossRef]
- Kortunov, P.V.; Siskin, M.; Baugh, L.S.; Calabro, D.C. In Situ Nuclear Magnetic Resonance Mechanistic Studies of Carbon Dioxide Reactions with Liquid Amines in Aqueous Systems: New Insights on Carbon Capture Reaction Pathways. Energy Fuels 2015, 29, 5919–5939. [Google Scholar] [CrossRef]
- Pinto, P.R.; Mendes, L.C.; Dias, M.L.; Azuma, C. Synthesis of Acrylic-Modified Sol–Gel Silica. Colloid. Polym. Sci. 2006, 284, 529–535. [Google Scholar] [CrossRef]
- Sandhu, N.K.; Pudasainee, D.; Sarkar, P.; Gupta, R. Steam Regeneration of Polyethylenimine-Impregnated Silica Sorbent for Postcombustion CO2 Capture: A Multicyclic Study. Ind. Eng. Chem. Res. 2016, 55, 2210–2220. [Google Scholar] [CrossRef]
- Banu Yener, H.; Helvacı, Ş.Ş. Effect of Synthesis Temperature on the Structural Properties and Photocatalytic Activity of TiO2/SiO2 Composites Synthesized Using Rice Husk Ash as a SiO2 Source. Sep. Purif. Technol. 2015, 140, 84–93. [Google Scholar] [CrossRef]
- Grenda, K.; Idström, A.; Evenäs, L.; Persson, M.; Holmberg, K.; Bordes, R. An Analytical Approach to Elucidate the Architecture of Polyethyleneimines. J. Appl. Polym. Sci. 2022, 139, 51657. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Arkas, M. Columnar and Smectic Self-Assembly Deriving from Non Ionic Amphiphilic Hyperbranched Polyethylene Imine Polymers and Induced by Hydrogen Bonding and Segregation into Polar and Non Polar Parts. Polymer 2013, 54, 1114–1122. [Google Scholar] [CrossRef]
- De Loos, M.; Van Esch, J.; Stokroos, I.; Kellogg, R.M.; Feringa, B.L. Remarkable Stabilization of Self-Assembled Organogels by Polymerization. J. Am. Chem. Soc. 1997, 119, 12675–12676. [Google Scholar] [CrossRef]
- Battjes, K.P.; Barolo, A.M.; Dreyfuss, P. New Evidence Related to Reactions of Aminated Silane Coupling Agents with Carbon Dioxide. J. Adhes. Sci. Technol. 1991, 5, 785–799. [Google Scholar] [CrossRef]
- Wilfong, W.C.; Srikanth, C.S.; Chuang, S.S.C. In Situ ATR and DRIFTS Studies of the Nature of Adsorbed CO2 on Tetraethylenepentamine Films. ACS Appl. Mater. Interfaces 2014, 6, 13617–13626. [Google Scholar] [CrossRef] [PubMed]
- Licari, D.; Baiardi, A.; Biczysko, M.; Egidi, F.; Latouche, C.; Barone, V. Implementation of a Graphical User Interface for the Virtual Multifrequency Spectrometer: The VMS-Draw Tool. J. Comput. Chem. 2015, 36, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Kummamuru, N.B.; Eimer, D.A.; Idris, Z. Viscosity Measurement and Correlation of Unloaded and CO2 -Loaded Aqueous Solutions of N-Methyldiethanolamine + 2-Amino-2-Methyl-1-Propanol. J. Chem. Eng. Data 2020, 65, 3072–3078. [Google Scholar] [CrossRef]
- Fu, D.; Wang, L.; Mi, C.; Zhang, P. Absorption Capacity and Viscosity for CO2 Capture Process Using High Concentrated PZ-DEAE Aqueous Solution. J. Chem. Thermodyn. 2016, 101, 123–129. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Dong, K.; Zhang, Y.; Shen, Y.; Lv, X. Supported Absorption of CO2 by Tetrabutylphosphonium Amino Acid Ionic Liquids. Chem. Eur. J. 2006, 12, 4021–4026. [Google Scholar] [CrossRef]
- Goodrich, B.F.; De La Fuente, J.C.; Gurkan, B.E.; Zadigian, D.J.; Price, E.A.; Huang, Y.; Brennecke, J.F. Experimental Measurements of Amine-Functionalized Anion-Tethered Ionic Liquids with Carbon Dioxide. Ind. Eng. Chem. Res. 2011, 50, 111–118. [Google Scholar] [CrossRef]
- Wang, L.; Al-Aufi, M.; Pacheco, C.N.; Xie, L.; Rioux, R.M. Polyethylene Glycol (PEG) Addition to Polyethylenimine (PEI)-Impregnated Silica Increases Amine Accessibility during CO2 Sorption. ACS Sustain. Chem. Eng. 2019, 7, 14785–14795. [Google Scholar] [CrossRef]
- Giorgini, M.G.; Pelletti, M.R.; Paliani, G.; Cataliotti, R.S. Vibrational Spectra and Assignments of Ethylene-Diamine and Its Deuterated Derivatives. J. Raman Spectrosc. 1983, 14, 16–21. [Google Scholar] [CrossRef]
- Von Harpe, A.; Petersen, H.; Li, Y.; Kissel, T. Characterization of Commercially Available and Synthesized Polyethylenimines for Gene Delivery. J. Control. Release 2000, 69, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Jeon, S.; Jo, D.H.; Huh, J.; Kim, S.H. Effect of Crosslinking on the CO2 Adsorption of Polyethyleneimine-Impregnated Sorbents. Chem. Eng. J. 2017, 307, 836–844. [Google Scholar] [CrossRef]
- Pierre, T.S.; Geckle, M. 13-NMR Analysis of Branched Polyethyleneimine. J. Macromol. Sci. Part. A-Chem. 1985, 22, 877–887. [Google Scholar] [CrossRef]
- Heydari-Gorji, A.; Sayari, A. Thermal, Oxidative, and CO2-Induced Degradation of Supported Polyethylenimine Adsorbents. Ind. Eng. Chem. Res. 2012, 51, 6887–6894. [Google Scholar] [CrossRef]
- Coralli, I.; Fabbri, D.; Facchin, A.; Torri, C.; Stevens, L.A.; Snape, C.E. Analytical Pyrolysis of Polyethyleneimines. J. Anal. Appl. Pyrolysis 2023, 169, 105838. [Google Scholar] [CrossRef]
- Vidović, N.; Antić, V.; Schwarzbauer, J. Determination of the Water-Soluble Polymer Poly(Ethyleneimine) (PEI) in Wastewater by Continuous-Flow off-Line Pyrolysis GC/MS. J. Anal. Appl. Pyrolysis 2023, 172, 106001. [Google Scholar] [CrossRef]
- Mortzfeld, F.B.; Hashem, C.; Vranková, K.; Winkler, M.; Rudroff, F. Pyrazines: Synthesis and Industrial Application of These Valuable Flavor and Fragrance Compounds. Biotechnol. J. 2020, 15, 2000064. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Woo, J.-M.; Jo, S.-H.; Lee, S.-Y.; Moon, J.-H.; Kim, H.; Yi, C.-K.; Lee, H.; Snape, C.E.; Stevens, L.; et al. Continuous Testing of Silica-PEI Adsorbents in a Lab.-Scale Twin Bubbling Fluidized-Bed System. Int. J. Greenh. Gas. Control 2019, 82, 184–191. [Google Scholar] [CrossRef]







| Sample | Char (%) |
|---|---|
| SP0 | 3.2 |
| S0 | 2.6 |
| SP2 | 5.1 |
| S2 | 2.8 |
| SP3 | 5.3 |
| S3 | 4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coralli, I.; Giuri, D.; Spada, L.; Ortolani, J.; Mazzocchetti, L.; Tomasini, C.; Stevens, L.A.; Snape, C.E.; Fabbri, D. Valorization Strategies in CO2 Capture: A New Life for Exhausted Silica-Polyethylenimine. Int. J. Mol. Sci. 2023, 24, 14415. https://doi.org/10.3390/ijms241914415
Coralli I, Giuri D, Spada L, Ortolani J, Mazzocchetti L, Tomasini C, Stevens LA, Snape CE, Fabbri D. Valorization Strategies in CO2 Capture: A New Life for Exhausted Silica-Polyethylenimine. International Journal of Molecular Sciences. 2023; 24(19):14415. https://doi.org/10.3390/ijms241914415
Chicago/Turabian StyleCoralli, Irene, Demetra Giuri, Lorenzo Spada, Jacopo Ortolani, Laura Mazzocchetti, Claudia Tomasini, Lee A. Stevens, Colin E. Snape, and Daniele Fabbri. 2023. "Valorization Strategies in CO2 Capture: A New Life for Exhausted Silica-Polyethylenimine" International Journal of Molecular Sciences 24, no. 19: 14415. https://doi.org/10.3390/ijms241914415