Genomic Factors and Therapeutic Approaches in HIV-Associated Neurocognitive Disorders: A Comprehensive Review
Abstract
:1. Introduction
1.1. Background and the Prevalence of HIV-Associated Neurocognitive Disorders
1.2. Diagnosis and Comorbidities
2. Important Genes Involved in the Development of HIV-Associated Neurocognitive Disorders
2.1. Genes Involved in Inflammation
2.2. Genes Related to Serotonin Neurotransmission
2.3. Involvement of Genes Related to Neurocognitive Disorders in Immune Regulation
2.4. Drug Metabolism and Transport Genes
2.5. Nuclear and Mitochondrial DNA Damage Genes
2.6. Matrix Metalloproteinase Genes
| Function | Genes Involved | References |
|---|---|---|
| Neuronal injury, neuroinflammation, and neurocognitive degeneration | soluble CD14 (sCD14) [13,17,18] soluble CD163 (sCD163) [13] macrophage chemo-attractant protein-1 (MCP-1) [16] neopterin [13,16,21] neurofilament light chain protein (NFL) [17] tumor necrosis factor-α (TNF-α) [16] macrophage inflammatory protein 1 (MCP-1/CCL2) [16] macrophage inflammatory protein 1-α (MIP-1α/CCL3) [16] stromal cell-derived factor-1 (SDF1) [16] mannose-binding lectin 2 (MBL2) [6,29,30,31,32,33] inducible nitric oxide synthase (iNOS) [6] surface CD16/32 [6] interleukin-6 (IL-6) [6] nitric oxide NO [16] interleukin-15 (IL-15) [19] C-Reactive Protein (CRP) [20] C-X-C Motif Chemokine Ligand 10 (CXCL10) [21] T lymphocytes (CTLs) [25]. HLA class I and HLA class II genes [25,26,27,28] | [6] Bhatia et al. (2014) https://doi.org/10.7860/JCDR/2014/7725.4927 [13] Jumare et al. (2020) https://doi.org/10.1097/QAI.0000000000002320 [16] Silveira et al. (2022) https://doi.org/10.1002/cbf.3685 [17] Anderson et al. (2020) https://doi.org/10.1097/QAI.0000000000002484 [19] Yan, J et al. (2021) https://doi.org/10.1002/cti2.1318 [20] Abu-Rumeileh et al. (2019) https://doi.org/10.1186/s13195-019-0562-4 [21] Khalil et al. (2018) https://doi.org/10.1038/s41582-018-0058-z [25] Olivier et al. (2018) https://doi.org/10.3390/ijms19113594 [26] Sanna et al. (2017) http://doi.org/10.1371/journal.pone.0175316 [27] Siangphoe et al. (2015) http://doi.org/10.1097/QAI.0000000000000800 [28] Sagar et al. (2017) http://doi.org/10.1371/journal.pone.0181642 [29] Singh et al. (2008 http://doi.org/10.1016/j.jaci.2008.05.025 [30] van Rij et al. (1999) http://doi.org/10.1086/314940 [31] Levine et al. (2016) http://doi.org/10.1007/s13365-015-0410-7 [32] Singh et al. (2011) http://doi.org/10.2147/NBHIV.S19969 [33] Jia et al. (2017) http://doi.org/10.1002/ajmg.b.32530 |
| Slower executive activity and memory and local release of serotonin in HIV+ patients | SNPs of serotonin-related genes: SNP rs4570625 [12,35] galactose mutarotase rs6741892 [35] | [12] Borrajo et al. (2021) https://doi.org/10.1080/07853890.2020.1814962 [35] Naranbhai et al. (2017) http://doi.org/10.1007/s00251-017-1000-z |
| Neurocognitive illness | Lipid metabolism: apolipoprotein Eε4 (ApoEε4) [40] Dopaminergic system: dopamine active transporter (DAT), Catechol-O-methyltransferase (COMT), Brain-derived neurotrophic factor (BDNF) [39,40] dopamine receptor D1, D2 and D3 (DRD1, DRD2, DRD3) [39,40,41] Immune system genes: C-C chemokine receptor type 5 and 2 (CCR5, CCR2) [44] C-C chemokine ligand type 2, 3 and 5 (CCL2, CCL3, CCL5) [44] | [39] Penedo et al. (2021). https://doi.org/10.1016/j.bbih.2021.100199 [40] Ojeda-Juárez et al. (2021 http://doi.org/10.3389/fmolb.2021.721954 [41] Gelman et al. (2012) https://doi.org/10.1007/s11481-012-9345-4 [35] [44] Borrajo et al. (2021). https://doi.org/10.3390/biomedicines9080925 |
| Neurocognitive impairment by drug metabolism and transport genes | Metabolizers based on polymorphisms: enzyme cytochrome P450, family 2, sub-family D, polypeptide 6 (CYP2D6) [14] enzyme cytochrome P450, family 2, sub-family C, polypeptide 19 (CYP2C19) [14] Lipid metabolism: apolipoprotein E3 and E4 (ApoE3 ApoE4) [14] | [14] Nordström et al. (2013) http://doi.org/10.1001/jamainternmed.2013.9079 |
| Oxidative impairment | Nuclear and mitochondrial DNA damage genes: nuclear DNA 8-oxoG [46] | [46] Malan-Müller et al. (2013) https://doi.org/10.1371/journal.pone.0058351 |
| Extracellular Matrix remodelling leading to blood-brainbarrier permeability damage | Matrix metalloproteinases (MMPs): matrix metalloproteinases 2 and 9 (MMP2, MMP9) [47,48,49,50] | [47] Bazzani et al. (2022) http://doi.org/10.3390/ijms231911391 [48] Xing et al. (2017) http://doi.org/10.1016/j.bbi.2017.04.024 [49] Singh et al. (2018) http://doi.org/10.1111/apm.12817 [50] Singh H et al. (2016) http://doi.org/10.1002/jgm.2897 |
3. Transcriptomic and Epigenetic Studies
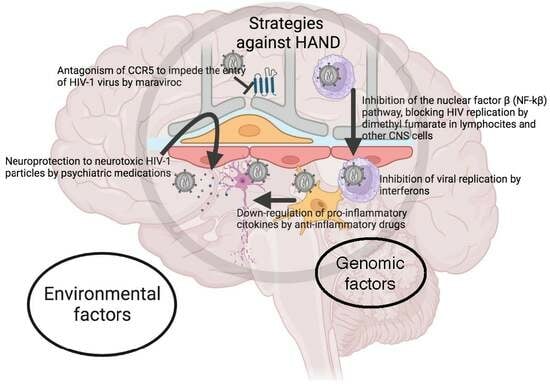
4. Current Evidence for Different Therapeutic Approaches
4.1. Antioxidative Drugs
4.2. Psychiatric Medications
4.3. Anti-Inflammatory Drugs
4.4. Antiretroviral Drugs
4.5. Interferons
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV infection. Nat. Rev. Dis. Prim. 2015, 1, 15035. [Google Scholar] [CrossRef] [PubMed]
- Bonfils, K.A.; Firmin, R.L.; Salyers, M.P.; Wright, E.R. Sexuality and intimacy among people living with serious mental illnesses: Factors contributing to sexual activity. Psychiatr. Rehabil. J. 2015, 38, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Remien, R.H.; Stirratt, M.J.; Nguyen, N.; Robbins, R.N.; Pala, A.N.; Mellins, C.A. Mental health and HIV/AIDS: The need for an integrated response. AIDS 2019, 33, 1411–1420. [Google Scholar] [CrossRef]
- Center for Behavioral Health Statistics and Quality. National Survey on Drug Use and Health: Methodological Summary and Definitions; Ubstance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2017.
- Kendall, C.E.; Wong, J.; Taljaard, M.; Glazier, R.H.; Hogg, W.; Younger, J.; Manuel, D.G. A cross-sectional, population-based study measuring comorbidity among people living with HIV in Ontario. BMC Public Health 2014, 14, 161. [Google Scholar] [CrossRef]
- Bhatia, M. Prevalence of Depression in People Living with HIV/AIDS Undergoing ART and Factors Associated with it. J. Clin. Diagn. Res. JCDR 2014, 8, WC01–WC04. [Google Scholar] [CrossRef]
- Niu, L.; Luo, D.; Liu, Y.; Silenzio, V.M.B.; Xiao, S. The Mental Health of People Living with HIV in China, 1998–2014: A Systematic Review. PLoS ONE 2016, 11, e0153489. [Google Scholar] [CrossRef] [PubMed]
- Tedaldi, E.M.; Minniti, N.L.; Fischer, T. HIV-Associated Neurocognitive Disorders: The Relationship of HIV Infection with Physical and Social Comorbidities. BioMed Res. Int. 2015, 2015, 641913. [Google Scholar] [CrossRef]
- Do, A.N.; Rosenberg, E.S.; Sullivan, P.S.; Beer, L.; Strine, T.W.; Schulden, J.D.; Fagan, J.L.; Freedman, M.S.; Skarbinski, J. Excess Burden of Depression among HIV-Infected Persons Receiving Medical Care in the United States: Data from the Medical Monitoring Project and the Behavioral Risk Factor Surveillance System. PLoS ONE 2014, 9, e92842. [Google Scholar] [CrossRef]
- Zhang, W.; O’brien, N.; Forrest, J.I.; Salters, K.A.; Patterson, T.L.; Montaner, J.S.G.; Hogg, R.S.; Lima, V.D. Validating a Shortened Depression Scale (10 Item CES-D) among HIV-Positive People in British Columbia, Canada. PLoS ONE 2012, 7, e40793. [Google Scholar] [CrossRef]
- Namagga, J.K.; Rukundo, G.Z.; Niyonzima, V.; Voss, J. Depression and HIV associated neurocognitive disorders among HIV infected adults in rural southwestern Uganda: A cross-sectional quantitative study. BMC Psychiatry 2021, 21, 350. [Google Scholar] [CrossRef]
- Borrajo, A.; Spuch, C.; Penedo, M.A.; Olivares, J.M.; Agís-Balboa, R.C. Important role of microglia in HIV-1 associated neurocognitive disorders and the molecular pathways implicated in its pathogenesis. Ann. Med. 2021, 53, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Jumare, J.M.; Akolo, C.M.; Ndembi, N.; Bwala, S.M.F.; Alabi, P.M.F.; Okwuasaba, K.M.; Adebiyi, R.; Umlauf, A.; Cherner, M.; Abimiku, A.; et al. Elevated Plasma Levels of sCD14 and MCP-1 Are Associated with HIV Associated Neurocognitive Disorders Among Antiretroviral-Naive Individuals in Nigeria. JAIDS J. Acquir. Immune Defic. Syndr. 2020, 84, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Nordström, P.; Nordström, A.; Eriksson, M.; Wahlund, L.-O.; Gustafson, Y. Risk Factors in Late Adolescence for Young-Onset Dementia in Men: A Nationwide Cohort Study. JAMA Intern. Med. 2013, 173, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Zenebe, Y.; Necho, M.; Yimam, W.; Akele, B. Worldwide Occurrence of HIV-Associated Neurocognitive Disorders and Its Associated Factors: A Systematic Review and Meta-Analysis. Front. Psychiatry 2022, 13, 814362. [Google Scholar] [CrossRef]
- Silveira, D.B.; Américo, M.F.; Flores, N.P.; Terenzi, H.; Pinto, A.R. Pharmacological inhibition of UPR sensor PERK attenuates HIV Tat-induced inflammatory M1 phenotype in microglial cells. Cell Biochem. Funct. 2022, 40, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.M.M.; Jang, J.H.; Easley, K.A.M.; Fuchs, D.; Gisslen, M.; Zetterberg, H.; Blennow, K.; Ellis, R.J.; Franklin, D.B.; Heaton, R.K.; et al. Cognitive and Neuronal Link with Inflammation: A Longitudinal Study in People With and Without HIV Infection. J. Acquir. Immune Defic. Syndr. (1999) 2020, 85, 617–625. [Google Scholar] [CrossRef]
- Rakshasa-Loots, A.M.; Whalley, H.C.; Vera, J.H.; Cox, S.R. Neuroinflammation in HIV-associated depression: Evidence and future perspectives. Mol. Psychiatry 2022, 27, 3619–3632. [Google Scholar] [CrossRef]
- Yan, J.; Kuzhiumparambil, U.; Bandodkar, S.; Dale, R.C.; Fu, S. Cerebrospinal fluid metabolomics: Detection of neuroinflammation in human central nervous system disease. Clin. Transl. Immunol. 2021, 10, e1318. [Google Scholar] [CrossRef]
- Abu-Rumeileh, S.; Steinacker, P.; Polischi, B.; Mammana, A.; Bartoletti-Stella, A.; Oeckl, P.; Baiardi, S.; Zenesini, C.; Huss, A.; Cortelli, P.; et al. CSF biomarkers of neuroinflammation in distinct forms and subtypes of neurodegenerative dementia. Alzheimer’s Res. Ther. 2019, 12, 2. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef]
- Rosadas, C.; Zetterberg, H.; Heslegrave, A.; Haddow, J.; Borisova, M.; Taylor, G.P. Neurofilament Light in CSF and Plasma Is a Marker of Neuronal Damage in HTLV-1–Associated Myelopathy and Correlates with Neuroinflammation. Neurol.-Neuroimmunol. Neuroinflamm. 2021, 8, e1090. [Google Scholar] [CrossRef] [PubMed]
- Conole, E.L.; Stevenson, A.J.; Maniega, S.M.; Harris, S.E.; Green, C.; Hernández, M.d.C.V.; Harris, M.A.; Bastin, M.E.; Wardlaw, J.M.; Deary, I.J.; et al. DNA Methylation and Protein Markers of Chronic Inflammation and Their Associations with Brain and Cognitive Aging. Neurology 2021, 97, e2340–e2352. [Google Scholar] [CrossRef] [PubMed]
- McLaren, P.J.; Fellay, J. HIV-1 and human genetic variation. Nat. Rev. Genet. 2021, 22, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Olivier, I.S.; Cacabelos, R.; Naidoo, V. Risk Factors and Pathogenesis of HIV-Associated Neurocognitive Disorder: The Role of Host Genetics. Int. J. Mol. Sci. 2018, 19, 3594. [Google Scholar] [CrossRef]
- Sanna, P.P.; Repunte-Canonigo, V.; Masliah, E.; Lefebvre, C. Gene expression patterns associated with neurological disease in human HIV infection. PLoS ONE 2017, 12, e0175316. [Google Scholar] [CrossRef]
- Siangphoe, U.; Archer, K.J. Gene expression in HIV-associated neurocognitive disorders: A meta-analysis. Acquir. Immune Defic. Syndr. 2015, 70, 479–488. [Google Scholar] [CrossRef]
- Sagar, V.; Pilakka-Kanthikeel, S.; Martinez, P.C.; Atluri, V.S.R.; Nair, M. Common gene-network signature of different neurological disorders and their potential implications to neuroAIDS. PLoS ONE 2017, 12, e0181642. [Google Scholar] [CrossRef]
- Singh, K.K.; Lieser, A.; Ruan, P.K.; Fenton, T.; Spector, S.A. An age-dependent association of mannose-binding lectin-2 genetic variants on HIV-1–related disease in children. J. Allergy Clin. Immunol. 2008, 122, 173–180.e2. [Google Scholar] [CrossRef]
- van Rij, R.P.; Portegies, P.; Hallaby, T.; Lange, J.M.; Visser, J.; de Roda Husman, A.M.; va not Wout, A.B.; Schuitemaker, H. Reduced Prevalence of the CCR5 Δ32 Heterozygous Genotype in Human Immunodeficiency Virus–Infected Individuals with AIDS Dementia Complex. J. Infect. Dis. 1999, 180, 854–857. [Google Scholar] [CrossRef]
- Levine, A.J.; Soontornniyomkij, V.; Achim, C.L.; Masliah, E.; Gelman, B.B.; Sinsheimer, J.S.; Singer, E.J.; Moore, D.J. Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J. NeuroVirology 2016, 22, 431–441. [Google Scholar] [CrossRef]
- Singh, K.K.; Nathamu, S.; Adame, A.; Alire, T.U.; Dumaop, W.; Gouaux, B.; Moore, D.J.; Masliah, E. Expression of mannose binding lectin in HIV-1-infected brain: Implications for HIV-related neuronal damage and neuroAIDS. Neurobehav. HIV Med. 2011, 3, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Zhao, Z.; Hulgan, T.; Bush, W.S.; Samuels, D.C.; Bloss, C.S.; Heaton, R.K.; Ellis, R.J.; Schork, N.; Marra, C.M.; et al. Genome-wide association study of HIV-associated neurocognitive disorder (HAND): A CHARTER group study. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 174, 413–426. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, K.A.; Harris, M.; Madormo, V.; Harrod, S.B.; Mactutus, C.F.; Booze, R.M. HIV-Associated Apathy/Depression and Neurocognitive Impairments Reflect Persistent Dopamine Deficits. Cells 2021, 10, 2158. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Carrington, M. Host genetic variation and HIV disease: From mapping to mechanism. Immunogenetics 2017, 69, 489–498. [Google Scholar] [CrossRef] [PubMed]
- López, A.B.; Penedo, M.A.; Rivera-Baltanas, T.; Pérez-Rodríguez, D.; Alonso-Crespo, D.; Fernández-Pereira, C.; Olivares, J.M.; Agís-Balboa, R.C. Microglia: The Real Foe in HIV-1-Associated Neurocognitive Disorders? Biomedicines 2021, 9, 925. [Google Scholar] [CrossRef]
- Horn, A.; Hiv/Aids, T.G.C.N.; Scheller, C.; du Plessis, S.; Burger, R.; Arendt, G.; Joska, J.; Sopper, S.; Maschke, C.M.; Obermann, M.; et al. The dopamine-related polymorphisms BDNF, COMT, DRD2, DRD3, and DRD4 are not linked with changes in CSF dopamine levels and frequency of HIV infection. J. Neural Transm. 2017, 124, 501–509. [Google Scholar] [CrossRef]
- Thames, A.D.; Briones, M.S.; Magpantay, L.I.; Martinez-Maza, O.; Singer, E.J.; Hinkin, C.H.; Morgello, S.; Gelman, B.B.; Moore, D.J.; Heizerling, K.; et al. The role of chemokine C-C motif ligand 2 genotype and cerebrospinal fluid chemokine C-C motif ligand 2 in neurocognition among HIV-infected patients. AIDS 2015, 29, 1483–1491. [Google Scholar] [CrossRef]
- Penedo, M.; Rivera-Baltanás, T.; Pérez-Rodríguez, D.; Allen, J.; Borrajo, A.; Alonso-Crespo, D.; Fernández-Pereira, C.; Nieto-Araujo, M.; Ramos-García, S.; Barreiro-Villar, C.; et al. The role of dopamine receptors in lymphocytes and their changes in schizophrenia. Brain Behav. Immun.-Health 2021, 12, 100199. [Google Scholar] [CrossRef]
- Ojeda-Juárez, D.; Kaul, M. Transcriptomic and Genetic Profiling of HIV-Associated Neurocognitive Disorders. Front. Mol. Biosci. 2021, 8, 721954. [Google Scholar] [CrossRef]
- Gelman, B.B.; Lisinicchia, J.G.; Chen, T.; Johnson, K.M.; Jennings, K.; Freeman, D.H.; Soukup, V.M. Prefrontal Dopaminergic and Enkephalinergic Synaptic Accommodation in HIV-associated Neurocognitive Disorders and Encephalitis. J. Neuroimmune Pharmacol. 2012, 7, 686–700. [Google Scholar] [CrossRef]
- Mediouni, S.; Marcondes, M.C.G.; Miller, C.; McLaughlin, J.P.; Valente, S.T. The cross-talk of HIV-1 Tat and methamphetamine in HIV-associated neurocognitive disorders. Front. Microbiol. 2015, 6, 1164. [Google Scholar] [CrossRef] [PubMed]
- Kallianpur, A.R.; Levine, A.J. Host Genetic Factors Predisposing to HIV-Associated Neurocognitive Disorder. Curr. HIV/AIDS Rep. 2014, 11, 336–352. [Google Scholar] [CrossRef] [PubMed]
- Borrajo, A.; Svicher, V.; Salpini, R.; Pellegrino, M.; Aquaro, S. Crucial Role of Central Nervous System as a Viral Anatomical Compartment for HIV-1 Infection. Microorganisms 2021, 9, 2537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, M.; Li, H.; Zhang, H.; Shi, Y.; Wei, F.; Liu, D.; Liu, K.; Chen, D. Accumulation of nuclear and mitochondrial DNA damage in the frontal cortex cells of patients with HIV-associated neurocognitive disorders. Brain Res. 2012, 1458, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Malan-Müller, S.; Hemmings, S.M.J.; Spies, G.; Kidd, M.; Fennema-Notestine, C.; Seedat, S. Shorter Telomere Length—A Potential Susceptibility Factor for HIV-Associated Neurocognitive Impairments in South African Woman. PLoS ONE 2013, 8, e58351. [Google Scholar] [CrossRef]
- Bazzani, V.; Redin, M.E.; McHale, J.; Perrone, L.; Vascotto, C. Mitochondrial DNA Repair in Neurodegenerative Diseases and Ageing. Int. J. Mol. Sci. 2022, 23, 11391. [Google Scholar] [CrossRef]
- Xing, Y.; Shepherd, N.; Lan, J.; Li, W.; Rane, S.; Gupta, S.K.; Zhang, S.; Dong, J.; Yu, Q. MMPs/TIMPs imbalances in the peripheral blood and cerebrospinal fluid are associated with the pathogenesis of HIV-1-associated neurocognitive disorders. Brain Behav. Immun. 2017, 65, 161–172. [Google Scholar] [CrossRef]
- Singh, H.; Samani, D.; Nambiar, N.; Ghate, M.V.; Gangakhedkar, R.R. Effect of matrix metalloproteinase-21 (572C/T) polymorphism on HIV-associated neurocognitive disorder. APMIS 2018, 126, 329–336. [Google Scholar] [CrossRef]
- Singh, H.; Marathe, S.D.; Nema, V.; Ghate, M.V.; Gangakhedkar, R.R. Genetic variation of MMP-2(-735 C>T) and MMP-9(-1562 C>T) gene in risk of development of HAND and severity of HAND. J. Gene Med. 2016, 18, 250–257. [Google Scholar] [CrossRef]
- Levine, A.J.; Panos, S.E.; Horvath, S. Genetic, Transcriptomic, and Epigenetic Studies of HIV-Associated Neurocognitive Disorder. J. Acquir. Immune Defic. Syndr. (1999) 2014, 65, 481–503. [Google Scholar] [CrossRef]
- Canchi, S.; Swinton, M.K.; Rissman, R.A.; Fields, J.A. Transcriptomic analysis of brain tissues identifies a role for CCAAT enhancer binding protein β in HIV-associated neurocognitive disorder. J. Neuroinflamm. 2020, 17, 112. [Google Scholar] [CrossRef] [PubMed]
- Gelman, B.B.; Chen, T.; Lisinicchia, J.G.; Soukup, V.M.; Carmical, J.R.; Starkey, J.M.; Masliah, E.; Commins, D.L.; Brandt, D.; Grant, I.; et al. The National NeuroAIDS Tissue Consortium Brain Gene Array: Two Types of HIV-Associated Neurocognitive Impairment. PLoS ONE 2012, 7, e46178. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, R.; Chang, J.R.; Del Valle, L.; Bagashev, A.; Gayed, M.M.; Lyde, R.B.; Hawkins, B.J.; Brailoiu, E.; Cohen, E.; Power, C.; et al. Deregulation of microRNAs by HIV-1 Vpr Protein Leads to the Development of Neurocognitive Disorders. J. Biol. Chem. 2011, 286, 34976–34985. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, D.; Agís-Balboa, R.C.; López-Fernández, H. MyBrain-Seq: A Pipeline for MiRNA-Seq Data Analysis in Neuropsychiatric Disorders. Biomedicines 2023, 11, 1230. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, D.; López-Fernández, H.; Agís-Balboa, R.C. Application of miRNA-seq in neuropsychiatry: A methodological perspective. Comput. Biol. Med. 2021, 135, 104603. [Google Scholar] [CrossRef]
- Xu, Z.; Asahchop, E.L.; Branton, W.G.; Gelman, B.B.; Power, C.; Hobman, T.C. MicroRNAs Upregulated during HIV Infection Target Peroxisome Biogenesis Factors: Implications for Virus Biology, Disease Mechanisms and Neuropathology. PLoS Pathog. 2017, 13, e1006360. [Google Scholar] [CrossRef]
- Saiyed, Z.M.; Gandhi, N.; Agudelo, M.; Napuri, J.; Samikkannu, T.; Reddy, P.V.; Khatavkar, P.; Yndart, A.; Saxena, S.K.; Nair, M.P. HIV-1 Tat upregulates expression of histone deacetylase-2 (HDAC2) in human neurons: Implication for HIV-associated neurocognitive disorder (HAND). Neurochem. Int. 2011, 58, 656–664. [Google Scholar] [CrossRef]
- Perez-Santiago, J.P.N.; Letendre, S.; Ellis, R.; Gouaux, B.; Moore, D.; LeBlanc, S.; Rajagopal, N.; Zhang, K.; Woelk, C. DNA methylation correlates with neurological decline in HIV-infected individuals. In Proceedings of the 19th Conference on Retroviruses and Opportunistic Infections, Seattle, WA, USA, 5–8 March 2012. [Google Scholar]
- Cross, S.A.; Cook, D.R.; Chi, A.W.S.; Vance, P.J.; Kolson, L.L.; Wong, B.J.; Jordan-Sciutto, K.L.; Kolson, D.L. Dimethyl Fumarate, an Immune Modulator and Inducer of the Antioxidant Response, Suppresses HIV Replication and Macrophage-Mediated Neurotoxicity: A Novel Candidate for HIV Neuroprotection. J. Immunol. 2011, 187, 5015–5025. [Google Scholar] [CrossRef]
- Linker, R.A.; Lee, D.-H.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011, 134 Pt 3, 678–692. [Google Scholar] [CrossRef]
- Meulendyke, K.A.; Queen, S.E.; Engle, E.L.; Shirk, E.N.; Liu, J.; Steiner, J.P.; Nath, A.; Tarwater, P.M.; Graham, D.R.; Mankowski, J.L.; et al. Combination fluconazole/paroxetine treatment is neuroprotective despite ongoing neuroinflammation and viral replication in an SIV model of HIV neurological disease. J. NeuroVirology 2014, 20, 591–602. [Google Scholar] [CrossRef]
- Sacktor, N.; Skolasky, R.L.; Moxley, R.; Wang, S.; Mielke, M.M.; Munro, C.; Steiner, J.; Nath, A.; Haughey, N.; McArthur, J. Paroxetine and fluconazole therapy for HIV-associated neurocognitive impairment: Results from a double-blind, placebo-controlled trial. J. NeuroVirology 2018, 24, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Omeragic, A.; Kayode, O.; Hoque, T.; Bendayan, R. Potential pharmacological approaches for the treatment of HIV-1 associated neurocognitive disorders. Fluids Barriers CNS 2020, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, T.; Jiang, W.; Hoque, T.; Henderson, J.; Wu, C.; Bendayan, R. Role of anti-inflammatory compounds in human immunodeficiency virus-1 glycoprotein120-mediated brain inflammation. J. Neuroinflamm. 2014, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.K.; Bowers, S.J.; Mitchell, R.J.; Potchoiba, M.J.; Schroeder, C.M.; Small, H.F. Preclinical assessment of the distribution of maraviroc to potential human immunodeficiency virus (HIV) sanctuary sites in the central nervous system (CNS) and gut-associated lymphoid tissue (GALT). Xenobiotica 2008, 38, 1330–1339. [Google Scholar] [CrossRef]
- Aquaro, S.; Borrajo, A.; Pellegrino, M.; Svicher, V. Mechanisms underlying of antiretroviral drugs in different cellular reservoirs with a focus on macrophages. Virulence 2020, 11, 400–413. [Google Scholar] [CrossRef]
- Thaney, V.E.; O’neill, A.M.; Hoefer, M.M.; Maung, R.; Sanchez, A.B.; Kaul, M. IFNβ Protects Neurons from Damage in a Murine Model of HIV-1 Associated Brain Injury. Sci. Rep. 2017, 7, srep46514. [Google Scholar] [CrossRef]
- Koneru, R.; Bimonte-Nelson, H.; Ciavatta, V.; Haile, W.; Elmore, K.; Ward, J.; Maroun, L.; Tyor, W.R. Reversing interferon-alpha neurotoxicity in a HIV-associated neurocognitive disorder mouse model. AIDS 2018, 32, 1403–1411. [Google Scholar] [CrossRef]
- Zhen, A.; Rezek, V.; Youn, C.; Lam, B.; Chang, N.; Rick, J.; Carrillo, M.; Martin, H.; Kasparian, S.; Syed, P.; et al. Targeting type I interferon–mediated activation restores immune function in chronic HIV infection. J. Clin. Investig. 2017, 127, 260–268. [Google Scholar] [CrossRef]
- Tang, Y.; Chaillon, A.; Gianella, S.; Wong, L.M.; Li, D.; Simermeyer, T.L.; Porrachia, M.; Ignacio, C.; Woodworth, B.; Zhong, D.; et al. Brain microglia serve as a persistent HIV reservoir despite durable antiretroviral therapy. J. Clin. Investig. 2023, 133, e167417. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrajo, A.; Pérez-Rodríguez, D.; Fernández-Pereira, C.; Prieto-González, J.M.; Agís-Balboa, R.C. Genomic Factors and Therapeutic Approaches in HIV-Associated Neurocognitive Disorders: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 14364. https://doi.org/10.3390/ijms241814364
Borrajo A, Pérez-Rodríguez D, Fernández-Pereira C, Prieto-González JM, Agís-Balboa RC. Genomic Factors and Therapeutic Approaches in HIV-Associated Neurocognitive Disorders: A Comprehensive Review. International Journal of Molecular Sciences. 2023; 24(18):14364. https://doi.org/10.3390/ijms241814364
Chicago/Turabian StyleBorrajo, Ana, Daniel Pérez-Rodríguez, Carlos Fernández-Pereira, José María Prieto-González, and Roberto Carlos Agís-Balboa. 2023. "Genomic Factors and Therapeutic Approaches in HIV-Associated Neurocognitive Disorders: A Comprehensive Review" International Journal of Molecular Sciences 24, no. 18: 14364. https://doi.org/10.3390/ijms241814364