Inhibition of NLRP3 Inflammasome Activation by 3H-1,2-Dithiole-3-Thione: A Potential Therapeutic Approach for Psoriasis Treatment
Abstract
:1. Introduction
2. Results
2.1. D3T Treatment Alleviated IMQ-Induced Psoriasis-like Skin Inflammation in BALB/c Mice
2.2. Histological Examination
2.3. D3T Could Decrease IL-17A Expression and Th17 Cell Differentiation
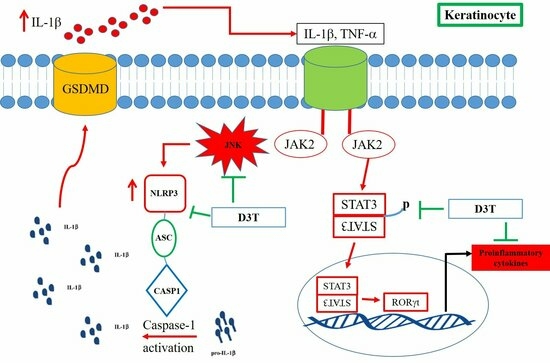
2.4. D3T Downregulating NLRP3 Inflammasome through Inhibit JNK Activation
3. Discussion
4. Materials and Methods
4.1. Mouse Model for Imiquimod (IMQ)-Induced Psoriasis-like Skin Inflammation
4.2. Histological and Immunohistochemical Stain
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Isolation of CD4+T Cells and Th17 Polarization
4.5. Flow Cytometry Analysis
4.6. Cell Culture
4.7. Immunoblotting
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michalek, I.; Loring, B.; John, S. A systematic review of worldwide epidemiology of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Pirowska, M.; Obtulowicz, A.; Lipko-Godlewska, S.; Gozdzialska, A.; Podolec, K.; Wojas-Pelc, A. The level of proinflammatory cytokines: Interleukins 12, 23, 17 and tumor necrosis factor alpha in patients with metabolic syndrome accompanying severe psoriasis and psoriatic arthritis. Postepy Dermatol. Alergol. 2018, 35, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhou, F. Inflammasomes in Common Immune-Related Skin Diseases. Front. Immunol. 2020, 11, 882. [Google Scholar] [CrossRef]
- Hooftman, A.; Angiari, S.; Hester, S.; Corcoran, S.E.; Runtsch, M.C.; Ling, C.; Ruzek, M.C.; Slivka, P.F.; McGettrick, A.F.; Banahan, K.; et al. The Immunomodulatory Metabolite Itaconate Modifies NLRP3 and Inhibits Inflammasome Activation. Cell Metab. 2020, 32, 468–478.e7. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Betharia, S.; Yen, J.-H.; Tran, Q.; Mistry, H.; Smith, K. Synthesis and structure–activity relationships study of dithiolethiones as inducers of glutathione in the SH-SY5Y neuroblastoma cell line. Bioorganic Med. Chem. Lett. 2014, 24, 5829–5831. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Z.; Zhang, L.; Yamamoto, M.; Misra, H.P.; Trush, M.A.; Li, Y. Antioxidants and Phase 2 Enzymes in Macrophages: Regulation by Nrf2 Signaling and Protection against Oxidative and Electrophilic Stress. Exp. Biol. Med. 2008, 233, 463–474. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Brown, D.A.; Scofield, B.A.; Yu, I.-C.; Chang, F.-L.; Wang, P.-Y.; Yen, J.-H. 3H-1,2-dithiole-3-thione as a novel therapeutic agent for the treatment of experimental autoimmune encephalomyelitis. Brain, Behav. Immun. 2016, 57, 173–186. [Google Scholar] [CrossRef]
- Hu, R.; Wang, M.Q.; Ni, S.H.; Wang, M.; Liu, L.Y.; You, H.Y.; Wu, X.H.; Wang, Y.J.; Lu, L.; Wei, L.B. Salidroside ameliorates endothelial inflammation and oxidative stress by regulating the AMPK/NF-κB/NLRP3 signaling pathway in AGEs-induced HUVECs. Eur. J. Pharmacol. 2020, 867, 172797. [Google Scholar] [CrossRef]
- Zhao, C.; Gu, Y.; Zeng, X.; Wang, J. NLRP3 inflammasome regulates Th17 differentiation in rheumatoid arthritis. Clin. Immunol. 2018, 197, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Wang, C.; Wu, Z.; Fan, L.; Tao, C.; Lin, J.; Chen, S.; Lin, Y.; Ge, Y. JNK/c-Jun-driven NLRP3 inflammasome activation in microglia contributed to retinal ganglion cells degeneration induced by indirect traumatic optic neuropathy. Exp. Eye Res. 2021, 202, 108335. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.-Y.; Lin, C.-H.; Sung, C.T.; Fang, J.-Y. Murine models of psoriasis and their usefulness for drug discovery. Expert Opin. Drug Discov. 2018, 13, 551–562. [Google Scholar] [CrossRef]
- Sharma, D.; Kanneganti, T.-D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Eigenbrod, T.; Muñoz-Planillo, R.; Nuñez, G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yao, Z. Roles of Infection in Psoriasis. Int. J. Mol. Sci. 2022, 23, 6955. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Xia, Y.; Huang, M.; Zhang, L.; Chen, L. Expression of NLPR3 in Psoriasis Is Associated with Enhancement of Interleukin-1β and Caspase-1. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 7909–7913. [Google Scholar] [CrossRef]
- Fitch, E.; Harper, E.; Skorcheva, I.; Kurtz, S.E.; Blauvelt, A. Pathophysiology of psoriasis: Recent advances on IL-23 and Th17 cytokines. Curr. Rheumatol. Rep. 2007, 9, 461–467. [Google Scholar] [CrossRef]
- Schon, M.P.; Erpenbeck, L. The Interleukin-23/Interleukin-17 Axis Links Adaptive and Innate Immunity in Psoriasis. Front. Immunol. 2018, 9, 1323. [Google Scholar] [CrossRef]
- Liu, C.T.; Yen, J.J.; Brown, D.A.; Song, Y.C.; Chu, M.Y.; Hung, Y.H.; Tang, Y.H.; Wu, P.Y.; Yen, H.R. Targeting Nrf2 with 3H-1,2-dithiole-3-thione to moderate OXPHOS-driven oxidative stress attenuates IL-17A-induced psoriasis. Biomed. Pharmacother. 2023, 159, 114294. [Google Scholar] [CrossRef]
- Haase, I.; Hobbs, R.M.; Romero, M.R.; Broad, S.; Watt, F.M. A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J. Clin. Investig. 2001, 108, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Furue, K.; Tsuji, G.; Nakahara, T. Interleukin-17A and Keratinocytes in Psoriasis. Int. J. Mol. Sci. 2020, 21, 1275. [Google Scholar] [CrossRef] [PubMed]
- Szentkereszty-Kovács, Z.; Gáspár, K.; Szegedi, A.; Kemény, L.; Kovács, D.; Törőcsik, D. Alcohol in Psoriasis—From Bench to Bedside. Int. J. Mol. Sci. 2021, 22, 4987. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Yuan, X.; Fang, H.; Chen, J.; Xue, K.; Li, Z.; Dang, E.; Wang, G.; Shao, S. Neutrophil extracellular traps promote keratinocyte inflammation via AIM2 inflammasome and AIM2-XIAP in psoriasis. Exp. Dermatol. 2023, 32, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.P.; Morrissey, R.L.; Tolhurst, T.A.; Crowell, J.A.; Levine, B.S. Oral Toxicity of 1,2-Dithiole-3-Thione, a Potential Cancer Chemopreventive Agent, in the Rat. Int. J. Toxicol. 2000, 19, 375–381. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, M.-C.; Li, C.-L.; Liao, E.-C.; Yen, C.-Y.; Yen, L.-J.; Wang, K.-C.; Lu, L.-Y.; Chou, T.-Y.; Chen, Y.-C.; Yu, S.-J. Inhibition of NLRP3 Inflammasome Activation by 3H-1,2-Dithiole-3-Thione: A Potential Therapeutic Approach for Psoriasis Treatment. Int. J. Mol. Sci. 2023, 24, 13528. https://doi.org/10.3390/ijms241713528
Shih M-C, Li C-L, Liao E-C, Yen C-Y, Yen L-J, Wang K-C, Lu L-Y, Chou T-Y, Chen Y-C, Yu S-J. Inhibition of NLRP3 Inflammasome Activation by 3H-1,2-Dithiole-3-Thione: A Potential Therapeutic Approach for Psoriasis Treatment. International Journal of Molecular Sciences. 2023; 24(17):13528. https://doi.org/10.3390/ijms241713528
Chicago/Turabian StyleShih, Meng-Chieh, Chia-Ling Li, En-Chih Liao, Chung-Yang Yen, Ling-Jung Yen, Kai-Chun Wang, Ling-Ying Lu, Ting-Yu Chou, Ying-Chin Chen, and Sheng-Jie Yu. 2023. "Inhibition of NLRP3 Inflammasome Activation by 3H-1,2-Dithiole-3-Thione: A Potential Therapeutic Approach for Psoriasis Treatment" International Journal of Molecular Sciences 24, no. 17: 13528. https://doi.org/10.3390/ijms241713528