A Comparative Analysis on the Innate Immune Responses of Cirrhinus mrigala Challenged with Pseudomonas aeruginosa and Fusarium oxysporum
Abstract
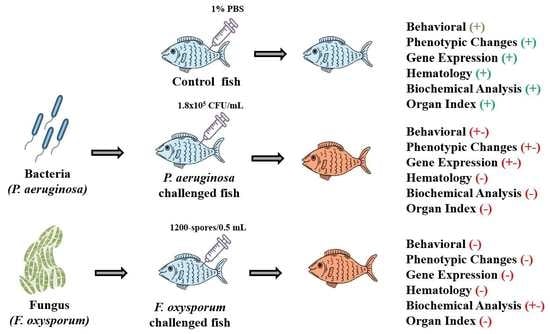
:1. Introduction
2. Results
2.1. Behavior and Phenotypic Alterations
2.2. Cytokine Expression Analysis
2.3. Hematological Analysis
2.4. Biochemical Analysis
2.5. Histological Examination
2.6. Organ Index Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Fish Rearing and Maintenance
4.2. P. aeruginosa Culture Preparation
4.3. Fungal Culture Preparation
4.4. Experimental Design
4.5. Cytokine Analysis
4.6. Hematological Studies
4.7. Biochemical Analysis
4.8. Histopathological Studies
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pervaiz, S.; Kanwal, Z.; Manzoor, F.; Tayyeb, A.; Akram, Q. Investigations on Blood Physiology, Tissues Histology and Gene Expression Profile of Fusarium oxysporum Challenged Fish. Sains Malays 2022, 51, 2403–2414. [Google Scholar] [CrossRef]
- Mastan, S.A. Pseudomonas septicaemia in Labeo rohita (Ham.) and Cyprinus carpio (Linn.) in Andhra Pradesh—Natural occurrence and artificial challenge. Int. J. Pharm. Pharm. Sci. 2013, 5, 564–568. [Google Scholar]
- Rohani, M.F.; Islam, S.M.; Hossain, M.K.; Ferdous, Z.; Siddik, M.A.; Nuruzzaman, M.; Padeniya, U.; Brown, C.; Shahjahan, M. Probiotics, Prebiotics and Synbiotics Improved the Functionality of Aquafeed: Upgrading Growth, Reproduction, Immunity and Disease Resistance in Fish. Fish Shellfish Immunol. 2022, 120, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; El-Ghiet, E.; Shaheen, A.; Abbass, A. Characterization of Pseudomonas species isolated from tilapia “Oreochromis niloticus” in Qaroun and Wadi-El-Rayan lakes, Egypt. Glob. Vet. 2010, 5, 116–121. [Google Scholar]
- Fry, J.P.; Love, D.C.; MacDonald, G.K.; West, P.C.; Engstrom, P.M.; Nachman, K.E.; Lawrence, R.S. Environmental health impacts of feeding crops to farmed fish. Environ. Int. 2016, 91, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Hanjra, M.A.; Blackwell, J.; Carr, G.; Zhang, F.; Jackson, T.M. Wastewater irrigation and environmental health: Implications for water governance and public policy. Int. J. Hyg. Environ. Health 2012, 215, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.B.; Mohd Khan, A.; Norrakiah, A.S. Microbiological risk assessment of fresh water aquaculture fish: From farm to table. Adv. Environ. Biol. 2014, 8, 105–111. [Google Scholar]
- Garrett, E.S.; Dos Santos, C.L.; Jahncke, M.L. Public, animal, and environmental health implications of aquaculture. Emerg. Infect. Dis. 1997, 3, 453–457. [Google Scholar] [CrossRef]
- Austin, B.; Austin, D.A. Bacterial Fish Pathogens, Disease of Farmed and Wild Fish, 4th ed.; Springer-Praxis: Chichester, UK, 2007. [Google Scholar]
- Kulatunga, D.C.M.; Dananjaya, S.H.S.; Park, B.K.; Kim, C.H.; Lee, J.; De Zoysa, M. First report of Fusarium oxysporum species complex infection in zebrafish culturing system. J. Fish Dis. 2017, 40, 485–494. [Google Scholar] [CrossRef]
- Bullock, G.L. Pseudomanadales as fish pathogens. Ind. Microbiol. 1964, 5, 101–108. [Google Scholar]
- Ukwe, O.; Oladapo-Akinfolarin, T. Alternations in Enzyme Activities of Clarias gariepinus Infected with Aeromonas hydrophila and Pseudomonas aeruginosa. Asian J. Fish. Aquat. Res. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wooster, G.A.; Bowser, P.R. Comparative blood chemistry and histopathology of tilapia infected with Virio vulnificus or Streptococcus iniae or exposed to carbon tetrachloride, gentamicin, or copper sulfate. Aquaculture 2004, 239, 421–443. [Google Scholar] [CrossRef]
- Moon, J.Y.; Kim, Y.B.; Lee, S.I.; Song, H.; Choi, K.; Jeong, G.H. Distribution characteristic of polychlorinated biphenylsincrucian carp (Carassiusauratusgibelio) from major rivers in Korea. Chemosphere 2006, 62, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Baloch, A.A.; Abdelsalam, E.E.E.; Piačková, V. Cytokines Studied in Carp (Cyprinus carpio L.) in Response to Important Diseases. Fishes 2021, 7, 3. [Google Scholar] [CrossRef]
- Panjvini, F.; Safoura, A.; Hossein, K.; Hossein, M.P. Parasitic infection alters hematology and immunity parameters of common carp, Cyprinus carpio. J. Parasit. Dis. 2016, 40, 1540–1543. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.L.; Altindag, A. Hematological parameters on tench (Tinca tinca L.) after acute and chronic exposure to lethal and sublthal mercury treatments. Bull. Environ. Contam. Toxicol. 2004, 73, 911–918. [Google Scholar] [CrossRef]
- Folmar, L.C. Effects of chemical contaminants on blood chemistry of teleost fish: A bibliography and synopsis of selected effects. Environ. Toxicol. Chem. 1993, 12, 337–375. [Google Scholar] [CrossRef]
- Figueiredo-Fernandes, A.; Ferreira-Cardoso, J.V.; Garcia-Santos, S.; Monteiro, S.M. Histopathological changes in liver and gill epithelium of Nile tilapia, Oreochromis niloticus, exposed to water borne copper. Pesq. Veter. Bras. 2007, 27, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Saikia, D.; Chattopadhyay, P.; Banerjee, G.; Sarma, D. Time and Dose Dependent Effect of Pseudomonas aeruginosa Infection on the Scales of Channa punctata (Bloch) Through Light and Electron Microscopy. Turk. J. Fish. Aquat. Sci. 2017, 17, 871–876. [Google Scholar] [CrossRef]
- Hassan, O.; Hassan, A.; Abd El Ghany, N.; El-baky, A.; Hanna, M. Abd El Aziz, M. A contribution on the pathogenicity of Fusarium oxysporum isolated from cultured Nile tilapia (Oreochromis niloticus) with trials for the treatment. Egypt. J. Aquat. Biol. Fish 2020, 24, 197–215. [Google Scholar] [CrossRef]
- Manoj, C.K.; Nair, C.; Patel, B.; Salin, R. Haematobiochemical and histopathological changes in Labeo rohita infected with Aeromonas hydrophila by immersion challenge. Fish. Technol. 2010, 47, 151–160. [Google Scholar]
- Amrevuawho, O.M.; Akinyemi, A.A.; Ezeri, O.G.; Bankole, O.M.; Takeet, O. Pathological study of Clarias gariepinus (Burchell, 1822) sub-adult artificially infected with Pseudomonas aeruginosa. Braz. J. Aquat. Sci. Technol. 2014, 18, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Salter, C.E.; Donnell, K.O.; Sutton, D.A.; Marancik, D.P.; Knowles, S.; Clauss, T.M.; Berliner, A.L.; Camus, A.C. Dermatitis and systemic mycosis in lined seahorses Hippocampus erectus associated with a marine-adapted Fusarium solani species complex pathogen. Dis. Aquat. Org. 2012, 101, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laing, K.J.; Wang, T.; Zou, J.; Holland, J.; Hong, S.; Bols, N.; Hirono, I.; Aoki, T.; Secombes, C.J. Cloning and expression analysis of rainbow trout Oncorhynchus mykiss tumour necrosis factor-α. Eur. J. Mol. Biol. Biochem. 2001, 268, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- So, T.; Lee, S.W.; Croft, M. Tumor necrosis factor/tumor necrosis factor receptor family members that positively regulate immunity. Int. J. Hematol. 2006, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.J.; Zhang, Z.P.; Lin, P.; Wang, S.H.; Zou, Z.H.; Wang, Y.L. Cloning and infection response of tumour-necrosis factor alpha in large yellow croaker Pseudosciaena crocea (Richardson). J. Fish Biol. 2008, 73, 1149–1160. [Google Scholar] [CrossRef]
- Syahputra, K.; Kania, P.W.; Al-Jubury, A.; Jafaar, R.M.; Dirks, R.P.; Buchmann, K. Transcriptomic analysis of immunity in rainbow trout (Oncorhynchus mykiss) gills infected by Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2019, 86, 486–496. [Google Scholar] [CrossRef]
- Tanekhy, M.; Matsuda, S.; Itano, T.; Kawakami, H.; Kono, T.; Sakai, M. Expression of cytokine genes in head kidney and spleen cells of Japanese flounder (Paralichthys olivaceus) infected with Nocardia seriolae. Vet. Immunol. Immunopathol. 2010, 134, 178–183. [Google Scholar] [CrossRef]
- Shah, S.L. Impairment in the haematological parameters of tench (Tinca tinca) infected by Saprolegnia spp. Turk. J. Vet. Anim. Sci. 2010, 34, 313–318. [Google Scholar] [CrossRef]
- Zaki, M.S.; Fawzi, O.M.; Jackey, J.E. Pathological and biochemical studies in Tilapia nilotica infected with Saprolegnia parasitica and treated with potassium permanganate. Am.-Eurasian J. Agric. Environ. Sci. 2008, 3, 677–680. [Google Scholar]
- Bektas, S.; Ayik, O. Hematological Parameters and Erythrocyte Osmotic Fragility in Rainbow Trout, Oncorhynchus mykiss, experimentally infected with Pseudomonas putida. J. Fish. Aquat. Sci. 2009, 4, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Perez-Rostro, C.I.; Racotta, I.S.; Ibarra, A.M. Decreased genetic variation in metabolic variables of Litopenaeus vannamei shrimp after exposure to acute hypoxia. J. Exp. Mar. Biol. Ecol. 2004, 302, 189–200. [Google Scholar] [CrossRef]
- Zayed, M.; Soliman, M.; Kalil, R.; Saad, T. Isolation of Candida albicans from naturally infected freshwater fish. Assiut. Vet. Med. J. 2016, 14, 21–45. [Google Scholar] [CrossRef]
- Shah, A.F.; Bhat, F.A.; Bhat, A.S.; Balkhi, M.H.; Abubakr, A.; Ahmad, I. Alteration in haemato-biochemical profiles of rainbow trout Oncorhynchus mykiss affected by Saprolegnia spp. A potential constraint for culture of trout in Kashmir Himalaya. Iran. J. Fish. Sci. 2015, 14, 970–984. [Google Scholar] [CrossRef]
- Qiao, G.; Park, S.; De-Hai, X. Clinical, Hematological, and Biochemical Alterations in Olive Flounder Paralichthys olivaceus Following Experimental Infection by Vibrio scophthalmi. Fish. Aquat. Sci. 2012, 15, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Saharia, P.; Pokhrel, H.; Kalita, B.; Hussain, I.A.; Islam, S. Histopathological changes in Indian Major Carp, Labeo rohita (Hamilton), experimentally infected with Aeromonas hydrophila associated with hemorrhagic septicemia of Central Brahmaputra valley of Assam. J. Entomol. Zool. Stud. 2018, 6, 6–11. [Google Scholar]
- El Sayyad, H.I.; Zaki, V.H.; El Shebby, A.M.; El Badry, D.A. Studies on the effect of bacteria diseases on the skin and gill structure of Clarias gariepinus in Dakahlia province, Egypt. Ann. Biol. Sci. 2010, 1, 106–118. [Google Scholar]
- Abdelhamed, H.; Ibrahim, I.; Baumgartner, W.; Lawrence, M.L.; Karsi, A. Characterization of Histopathological and Ultrastructural Changes in Channel Catfish Experimentally Infected with Virulent Aeromonas hydrophila. Front. Microbiol. 2017, 1, 1519. [Google Scholar] [CrossRef]
- Stratev, D.; Stoev, S.; Vashin, I. Some varieties of pathological changes in eximentalper infection of carps (Cyprinus carpio) with Aeromonas hydrophila. J. Aquac. Eng. Fish. Res. 2015, 1, 191–202. [Google Scholar] [CrossRef]
- Laith, A.R.; Najiah, M. Aeromonas hydrophila: Antimicrobial Susceptibility and Histopathology of Isolates from Diseased Catfish, Clarias gariepinus (Burchell). J. Aquac. Res. Dev. 2014, 5, 215. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.C.; Hsu, P.C.; Jen, C.F.; Chen, I.H.; Wang, C.H.; Chan, H.C.; Tsai, P.W.; Tung, K.C.; Wang, C.H.; Lan, C.Y.; et al. Zebrafish as a model host for Candida albicans infection. Infect. Immun. 2010, 78, 2512–2521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Covello, J.M.; Bird, S.; Morrison, R.N.; Battaglene, S.C.; Secombes, C.J.; Nowak, B.F. Cloning and expression analysis of three striped trumpeter (Latris lineata) pro-inflammatory cytokines, TNF-a, IL-1b and IL-8, in response to infection by the ectoparasitic, Chondracanthus goldsmidi. Fish Shellfish Immunol. 2009, 26, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzzaman, M.; Hossain, M.D.; Hossain, I. Antifungal and morphological assay of selective Trichoderma isolates against soil borne plant pathogenic fungi. Int. J. Innov. Appl. Stud. 2016, 16, 409. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.B.; Swain, N.K.; Maiti, P.; Routray, M.S. Inductive expression of toll-like receptor 5 (TLR5) and associated downstream signaling molecules following ligand exposure and bacterial infection in the Indian major carp, mrigal (Cirrhinus mrigala). Fish Shellfish Immunol. 2012, 32, 121–131. [Google Scholar] [CrossRef]
- Swain, B.; Basu, M.; Samanta, M. NOD1 and NOD2 receptors in Mrigal (Cirrhinus mrigala): Inductive expression and downstream signaling in ligand stimulation and bacterial infections. J. Biosci. 2013, 38, 533–548. [Google Scholar] [CrossRef]
- Mohanty, B.R.; Sahoo, P.K. Immune responses and expression profiles of some immune-related genes in Indian major carp, Labeo rohita to Edwardsiella tarda infection. Fish Shellfish Immunol. 2010, 28, 613–621. [Google Scholar] [CrossRef]





| Categories of Behavior | Pattern of Behavior | Control | P. aeruginosa | F. oxysporum |
|---|---|---|---|---|
| General activity |
| + | - | - |
| + | - | +- | |
| + | - | - | |
| Interaction |
| + | - | - |
| + | +- | - | |
| Maintenance behavior |
| + | +- | - |
| Control | P. aeruginosa | F. oxysporum | |
|---|---|---|---|
| Gills | 0.050 ± 0.028 a | 16.65 ± 0.638 b | 17.95 ± 0.232 c |
| Liver | 0.167± 0.145 b | 24.50 ± 0.248 b | 23.13 ± 0.478 c |
| Kidney | 0.052 ± 0.017 a | 23.00 ± 0.714 b | 28.33 ± 0.356 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, Z.; Kanwal, Z.; Tayyeb, A.; Noshair, I.; Haider, I.; Ahmad, N.; Alomar, S.Y. A Comparative Analysis on the Innate Immune Responses of Cirrhinus mrigala Challenged with Pseudomonas aeruginosa and Fusarium oxysporum. Int. J. Mol. Sci. 2023, 24, 12392. https://doi.org/10.3390/ijms241512392
Usman Z, Kanwal Z, Tayyeb A, Noshair I, Haider I, Ahmad N, Alomar SY. A Comparative Analysis on the Innate Immune Responses of Cirrhinus mrigala Challenged with Pseudomonas aeruginosa and Fusarium oxysporum. International Journal of Molecular Sciences. 2023; 24(15):12392. https://doi.org/10.3390/ijms241512392
Chicago/Turabian StyleUsman, Zaeema, Zakia Kanwal, Asima Tayyeb, Iqra Noshair, Imran Haider, Naushad Ahmad, and Suliman Yousef Alomar. 2023. "A Comparative Analysis on the Innate Immune Responses of Cirrhinus mrigala Challenged with Pseudomonas aeruginosa and Fusarium oxysporum" International Journal of Molecular Sciences 24, no. 15: 12392. https://doi.org/10.3390/ijms241512392