Irisin Is Target of Sphingosine-1-Phosphate/Sphingosine-1-Phosphate Receptor-Mediated Signaling in Skeletal Muscle Cells
Abstract
:1. Introduction
2. Results
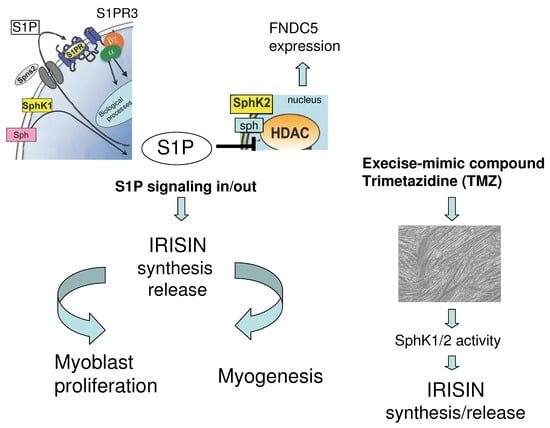
2.1. Effect of S1P Metabolism on Irisin Release and FNDC5 Expression in C2C12 Terminally Differentiated Myotubes
2.2. S1P Modulates Irisin-Induced Cell Proliferation and Myogenic Differentiation
2.3. Irisin Release Requires S1PR-Mediated Cytoskeleton Remodeling in Myotubes
2.4. S1P Modulates the Secretion of Irisin Promoted by Trimetazidine
3. Discussion
3.1. Inhibition of SphK2 and S1P Lyase, but Not SphK1, Affects FNDC5 Expression in Myotubes
3.2. Irisin Secretion Depends on S1P Accumulation and S1PR Signalling in Myotubes
3.3. Functional Crosstalk between Irisin and S1P in Skeletal Muscle Cells
4. Materials and Methods
4.1. Materials Biochemicals, Cell Culture Reagents
4.2. Cell Culture and Treatments
4.3. Enzyme-Linked Immunosorbent Assay (ELISA) Analysis of Irisin Concentration in C2C12 Myotubes Culture Medium
4.4. Cell Proliferation, Viability and Morphological Analyses
4.5. Quantification of RNA and Expression by Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.6. Western Blot Analysis
4.7. Confocal Laser Scanning Microscope Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, bnaa016. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A.; Pedersen, B.K. Who would have thought—Myokines two decades on. Nat. Rev. Endocrinol. 2020, 16, 619–620. [Google Scholar] [CrossRef]
- Mancinelli, R.; Checcaglini, F.; Coscia, F.; Gigliotti, P.; Fulle, S.; Fanò-Illic, G. Biological Aspects of Selected Myokines in Skeletal Muscle: Focus on Aging. Int. J. Mol. Sci. 2021, 22, 8520. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cui, F.; Ning, K.; Wang, Z.; Fu, P.; Wang, D.; Xu, H. Role of irisin in physiology and pathology. Front. Endocrinol. 2022, 13, 95–106. [Google Scholar] [CrossRef]
- Lapa, C.; Arias-Loza, P.; Hayakawa, N.; Wakabayashi, H.; Werner, R.A.; Chen, X.; Shinaji, T.; Herrmann, K.; Pelzer, T.; Higuchi, T. Whitening and Impaired Glucose Utilization of Brown Adipose Tissue in a Rat Model of Type 2 Diabetes Mellitus. Sci. Rep. 2017, 7, 7685. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Corleo, D.; Buscemi, C.; Giordano, C. Does iris(in) bring bad news or good news? Eat. Weight. Disord. 2018, 23, 431–442. [Google Scholar] [CrossRef]
- Abedi-Taleb, E.; Vahabi, Z.; Sekhavati-Moghadam, E.; Khedmat, L.; Jazayeri, S.; Saboor-Yaraghi, A.A. Upregulation of FNDC5 gene expression in C2C12 cells after single and combined treatments of resveratrol and ATRA. Lipids Health Dis. 2019, 18, 181. [Google Scholar] [CrossRef]
- Young, M.F.; Valaris, S.; Wrann, C.D. A role for FNDC5/Irisin in the beneficial effects of exercise on the brain and in neurodegenerative diseases. Prog. Cardiovasc. Dis. 2019, 62, 172–178. [Google Scholar] [CrossRef]
- Bao, J.-F.; She, Q.-Y.; Hu, P.-P.; Jia, N.; Li, A. Irisin, a fascinating field in our times. Trends Endocrinol. Metab. 2022, 33, 601–613. [Google Scholar] [CrossRef]
- Paris, M.T.; Bell, K.E.; Mourtzakis, M. Myokines and adipokines in sarcopenia: Understanding cross-talk between skeletal muscle and adipose tissue and the role of exercise. Curr. Opin. Pharmacol. 2020, 52, 61–66. [Google Scholar] [CrossRef]
- da Silva Lage, V.K.; de Paula, F.A.; Lima, L.P.; Santos, J.N.V.; Dos Santos, J.M.; Viegas, Â.A.; da Silva, G.P.; de Almeida, H.C.; da Silva Nunes Teixeira Rodrigues, A.L.; Leopoldino, A.A.O.; et al. Plasma levels of myokines and inflammatory markers are related with functional and respiratory performance in older adults with COPD and sarcopenia. Exp. Gerontol. 2022, 164, 111834. [Google Scholar] [CrossRef] [PubMed]
- Farshbaf, M.J.; Alviña, K. Multiple Roles in Neuroprotection for the Exercise Derived Myokine Irisin. Front. Aging Neurosci. 2021, 13, 649929. [Google Scholar] [CrossRef]
- Colaianni, G.; Cinti, S.; Colucci, S.; Grano, M. Irisin and musculoskeletal health. Ann. N. Y. Acad. Sci. 2017, 1402, 5–9. [Google Scholar] [CrossRef]
- Lavi, G.; Horwitz, A.; Einstein, O.; Zipori, R.; Gross, O.; Birk, R. Fndc5/irisin is regulated by myogenesis stage, irisin, muscle type and training. Am. J. Transl. Res. 2022, 14, 7063–7079. [Google Scholar]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int. J. Obes. 2014, 38, 1538–1544. [Google Scholar] [CrossRef]
- Rodríguez, A.; Becerril, S.; Méndez-Giménez, L.; Ramírez, B.; Sáinz, N.; Catalán, V.; Gómez-Ambrosi, J.; Frühbeck, G. Leptin administration activates irisin-induced myogenesis via nitric oxide-dependent mechanisms, but reduces its effect on subcutaneous fat browning in mice. Int. J. Obes. 2014, 39, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Kong, I.D. Irisin prevents dexamethasone-induced atrophy in C2C12 myotubes. Pflug. Arch.—Eur. J. Physiol. 2020, 472, 495–502. [Google Scholar] [CrossRef]
- Olivera, A.; Allende, M.L.; Proia, R.L. Shaping the landscape: Metabolic regulation of S1P gradients. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2013, 1831, 193–202. [Google Scholar] [CrossRef]
- Meacci, E.; Nuti, F.; Donati, C.; Cencetti, F.; Farnararo, M.; Bruni, P. Sphingosine kinase activity is required for myogenic differentiation of C2C12 myoblasts. J. Cell. Physiol. 2007, 214, 210–220. [Google Scholar] [CrossRef]
- Sassoli, C.; Frati, A.; Tani, A.; Anderloni, G.; Pierucci, F.; Matteini, F.; Chellini, F.; Orlandini, S.Z.; Formigli, L.; Meacci, E. Mesenchymal Stromal Cell Secreted Sphingosine 1-Phosphate (S1P) Exerts a Stimulatory Effect on Skeletal Myoblast Proliferation. PLoS ONE 2014, 9, e108662. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 19, 175–191. [Google Scholar] [CrossRef]
- Albeituni, S.; Stiban, J. Roles of Ceramides and Other Sphingolipids in Immune Cell Function and Inflammation. Adv. Exp. Med. Biol. 2019, 1161, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Fugio, L.B.; Coeli-Lacchini, F.B.; Leopoldino, A.M. Sphingolipids and Mitochondrial Dynamic. Cells 2020, 9, 581. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Okazaki, T. Ceramide/Sphingomyelin Rheostat Regulated by Sphingomyelin Synthases and Chronic Diseases in Murine Models. J. Lipid Atheroscler. 2020, 9, 380. [Google Scholar] [CrossRef]
- Chakraborty, P.; Vaena, S.G.; Thyagarajan, K.; Chatterjee, S.; Al-Khami, A.; Selvam, S.P.; Nguyen, H.; Kang, I.; Wyatt, M.W.; Baliga, U.; et al. Pro-Survival Lipid Sphingosine-1-Phosphate Metabolically Programs T Cells to Limit Anti-tumor Activity. Cell Rep. 2019, 28, 1879–1893.e7. [Google Scholar] [CrossRef] [PubMed]
- Presa, N.; Gomez-Larrauri, A.; Dominguez-Herrera, A.; Trueba, M.; Gomez-Muñoz, A. Novel signaling aspects of ceramide 1-phosphate. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2020, 1865, 158630. [Google Scholar] [CrossRef] [PubMed]
- Vestri, A.; Pierucci, F.; Frati, A.; Monaco, L.; Meacci, E. Sphingosine 1-Phosphate Receptors: Do They Have a Therapeutic Potential in Cardiac Fibrosis? Front. Pharmacol. 2017, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Meacci, E.; Garcia-Gil, M.; Pierucci, F. SARS-CoV-2 Infection: A Role for S1P/S1P Receptor Signaling in the Nervous System? Int. J. Mol. Sci. 2020, 21, 6773. [Google Scholar] [CrossRef]
- Pierucci, F.; Frati, A.; Battistini, C.; Matteini, F.; Iachini, M.C.; Vestri, A.; Penna, F.; Costelli, P.; Meacci, E. Involvement of released sphingosine 1-phosphate/sphingosine 1-phosphate receptor axis in skeletal muscle atrophy. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2018, 1864, 3598–3614. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Formigli, L.; Bini, F.; Tani, A.; Squecco, R.; Battistini, C.; Zecchi-Orlandini, S.; Francini, F.; Meacci, E. Effects of S1P on skeletal muscle repair/regeneration during eccentric contraction. J. Cell. Mol. Med. 2011, 15, 2498–2511. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Pierucci, F.; Zecchi-Orlandini, S.; Meacci, E. Sphingosine 1-Phosphate (S1P)/S1P Receptor Signaling and Mechanotransduction: Implications for Intrinsic Tissue Repair/Regeneration. Int. J. Mol. Sci. 2019, 20, 5545. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.V.; Silva, V.R.R.; Pauli, J.R.; da Silva, A.S.R.; Cintra, D.E.; Moura, L.P.; Ropelle, E.R. The role of sphingosine-1-phosphate in skeletal muscle: Physiology, mechanisms, and clinical perspectives. J. Cell. Physiol. 2018, 234, 10047–10059. [Google Scholar] [CrossRef] [PubMed]
- Meacci, E.; Vasta, V.; Donati, C.; Farnararo, M.; Bruni, P. Receptor-mediated activation of phospholipase D by sphingosine 1-phosphate in skeletal muscle C2C12 cells. FEBS Lett. 1999, 457, 184–188. [Google Scholar] [CrossRef]
- Meacci, E.; Cencetti, F.; Formigli, L.; Squecco, R.; Donati, C.; Tiribilli, B.; Quercioli, F.; Orlandini, S.Z.; Francini, F.; Bruni, P. Sphingosine 1-phosphate evokes calcium signals in C2C12 myoblasts via Edg3 and Edg5 receptors. Biochem. J. 2002, 362, 349. [Google Scholar] [CrossRef] [PubMed]
- Squecco, R.; Sassoli, C.; Nuti, F.; Martinesi, M.; Chellini, F.; Nosi, D.; Zecchi-Orlandini, S.; Francini, F.; Formigli, L.; Meacci, E. Sphingosine 1-Phosphate Induces Myoblast Differentiation through Cx43 Protein Expression: A Role for a Gap Junction-dependent and-independent Function. Mol. Biol. Cell 2006, 17, 4896–4910. [Google Scholar] [CrossRef]
- Formigli, L.; Meacci, E.; Sassoli, C.; Squecco, R.; Nosi, D.; Chellini, F.; Naro, F.; Francini, F.; Zecchi-Orlandini, S. Cytoskeleton/stretch-activated ion channel interaction regulates myogenic differentiation of skeletal myoblasts. J. Cell. Physiol. 2007, 211, 296–306. [Google Scholar] [CrossRef]
- Meacci, E.; Bini, F.; Sassoli, C.; Martinesi, M.; Squecco, R.; Chellini, F.; Zecchi-Orlandini, S.; Francini, F.; Formigli, L. Functional interaction between TRPC1 channel and connexin-43 protein: A novel pathway underlying S1P action on skeletal myogenesis. Cell. Mol. Life Sci. 2010, 67, 4269–4285. [Google Scholar] [CrossRef]
- Danieli-Betto, D.; Germinario, E.; Esposito, A.; Megighian, A.; Midrio, M.; Ravara, B.; Damiani, E.; Libera, L.D.; Sabbadini, R.A.; Betto, R. Sphingosine 1-phosphate protects mouse extensor digitorum longus skeletal muscle during fatigue. Am. J. Physiol. Physiol. 2005, 288, C1367–C1373. [Google Scholar] [CrossRef]
- Ieronimakis, N.; Pantoja, M.; Hays, A.L.; Dosey, T.L.; Qi, J.; Fischer, K.A.; Hoofnagle, A.N.; Sadilek, M.; Chamberlain, J.S.; Ruohola-Baker, H.; et al. Increased sphingosine-1-phosphate improves muscle regeneration in acutely injured mdx mice. Skelet. Muscle 2013, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, F.; Frati, A.; Battistini, C.; Penna, F.; Costelli, P.; Meacci, E. Control of Skeletal Muscle Atrophy Associated to Cancer or Corticosteroids by Ceramide Kinase. Cancers 2021, 13, 3285. [Google Scholar] [CrossRef] [PubMed]
- Blaho, V.A.; Hla, T. An update on the biology of sphingosine 1-phosphate receptors. J. Lipid Res. 2014, 55, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Meacci, E. Down-regulation of EDG5/S1P2 during myogenic differentiation results in the specific uncoupling of sphingosine 1-phosphate signalling to phospholipase D. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2003, 1633, 133–142. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Yao, W.J. Role of Rho/ROCK in the migration of vascular smooth muscle cells. Sheng Li Ke Xue Jin Zhan 2013, 44, 269–274. [Google Scholar]
- Maeda, Y.; Yagi, H.; Takemoto, K.; Utsumi, H.; Fukunari, A.; Sugahara, K.; Masuko, T.; Chiba, K. S1P lyase in thymic perivascular spaces promotes egress of mature thymocytes via up-regulation of S1P receptor 1. Int. Immunol. 2014, 26, 245–255. [Google Scholar] [CrossRef]
- French, K.J.; Schrecengost, R.S.; Lee, B.D.; Zhuang, Y.; Smith, S.N.; Eberly, J.L.; Yun, J.; Smith, C.D. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003, 63, 5962–5969. [Google Scholar]
- Schnute, M.E.; McReynolds, M.D.; Carroll, J.; Chrencik, J.; Highkin, M.K.; Iyanar, K.; Jerome, G.; Rains, J.W.; Saabye, M.; Scholten, J.A.; et al. Discovery of a Potent and Selective Sphingosine Kinase 1 Inhibitor through the Molecular Combination of Chemotype-Distinct Screening Hits. J. Med. Chem. 2017, 60, 2562–2572. [Google Scholar] [CrossRef]
- Venant, H.; Rahmaniyan, M.; Jones, E.E.; Lu, P.; Lilly, M.B.; Garrett-Mayer, E.; Drake, R.R.; Kraveka, J.M.; Smith, C.D.; Voelkel-Johnson, C. The Sphingosine Kinase 2 Inhibitor ABC294640 Reduces the Growth of Prostate Cancer Cells and Results in Accumulation of Dihydroceramides In Vitro and In Vivo. Mol. Cancer Ther. 2015, 14, 2744–2752. [Google Scholar] [CrossRef]
- Companioni, O.; Mir, C.; Garcia-Mayea, Y.; LLeonart, M.E. Targeting Sphingolipids for Cancer Therapy. Front. Oncol. 2021, 11, 745092. [Google Scholar] [CrossRef]
- Meacci, E.; Bini, F.; Battistini, C. Sphingosine-1-phosphate signaling in skeletal muscle cells. Methods Mol. Biol. 2012, 874, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; McNaughton, M.; Boomkamp, S.; MacRitchie, N.; Evangelisti, C.; Martelli, A.M.; Jiang, H.-R.; Ubhi, S.; Pyne, S. Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation. Adv. Biol. Regul. 2016, 60, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Formigli, L. Sphingosine 1-phosphate induces cytoskeletal reorganization in C2C12 myoblasts: Physiological relevance for stress fibres in the modulation of ion current through stretch-activated channels. J. Cell Sci. 2005, 118, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Nakata, J.; Akiba, Y.; Nihara, J.; Thant, L.; Eguchi, K.; Kato, H.; Izumi, K.; Ohkura, M.; Otake, M.; Kakihara, Y.; et al. ROCK inhibitors enhance bone healing by promoting osteoclastic and osteoblastic differentiation. Biochem. Biophys. Res. Commun. 2020, 526, 547–552. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Jing, X.; Liu, Y.; Liu, B.; She, Q. Sphingosine 1-phosphate induces epicardial progenitor cell differentiation into smooth muscle-like cells. Acta Biochim. Biophys. Sin. 2019, 51, 402–410. [Google Scholar] [CrossRef]
- Song, M.; Chen, F.-F.; Li, Y.-H.; Zhang, L.; Wang, F.; Qin, R.-R.; Wang, Z.-H.; Zhong, M.; Tang, M.-X.; Zhang, W.; et al. Trimetazidine restores the positive adaptation to exercise training by mitigating statin-induced skeletal muscle injury. J. Cachexia Sarcopenia Muscle 2017, 9, 106–118. [Google Scholar] [CrossRef]
- Xie, M.; Jiang, L.; Dun, Y.; Zhang, W.; Liu, S. Trimetazidine combined with exercise improves exercise capacity and anti-fatal stress ability through enhancing mitochondrial quality control. Life Sci. 2019, 224, 157–168. [Google Scholar] [CrossRef]
- Ding, G.; Sonoda, H.; Yu, H.; Kajimoto, T.; Goparaju, S.K.; Jahangeer, S.; Okada, T.; Nakamura, S.-I. Protein Kinase D-mediated Phosphorylation and Nuclear Export of Sphingosine Kinase 2. J. Biol. Chem. 2007, 282, 27493–27502. [Google Scholar] [CrossRef]
- Strub, G.M.; Paillard, M.; Liang, J.; Gomez, L.; Allegood, J.C.; Hait, N.C.; Maceyka, M.; Price, M.M.; Chen, Q.; Simpson, D.C.; et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2010, 25, 600–612. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, C.; Liu, P.; Zhao, J.; Xu, W. Sphingosine 1-phosphate (S1P) promotes mitochondrial biogenesis in Hep G2 cells by activating Peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α). Cell Stress Chaperones 2013, 19, 541–548. [Google Scholar] [CrossRef]
- Lima, S.; Milstien, S.; Spiegel, S. Sphingosine and Sphingosine Kinase 1 Involvement in Endocytic Membrane Trafficking. J. Biol. Chem. 2017, 292, 3074–3088. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of Histone Acetylation in the Nucleus by Sphingosine-1-Phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Jeong, Y.J.; Song, I.-S.; Noh, Y.H.; Seo, K.W.; Kim, M.; Han, J. Glucocorticoid receptor positively regulates transcription of FNDC5 in the liver. Sci. Rep. 2017, 7, srep43296. [Google Scholar] [CrossRef] [PubMed]
- Escarcega, R.D.; McCullough, L.D.; Tsvetkov, A.S. The Functional Role of Sphingosine Kinase 2. Front. Mol. Biosci. 2021, 8, 683767. [Google Scholar] [CrossRef] [PubMed]
- Siow, D.L.; Anderson, C.D.; Berdyshev, E.V.; Skobeleva, A.; Pitson, S.M.; Wattenberg, B.W. Intracellular localization of sphingosine kinase 1 alters access to substrate pools but does not affect the degradative fate of sphingosine-1-phosphate. J. Lipid Res. 2010, 51, 2546–2559. [Google Scholar] [CrossRef]
- Zhao, R. Irisin at the crossroads of inter-organ communications: Challenge and implications. Front. Endocrinol. 2022, 13, 989135. [Google Scholar] [CrossRef]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Eren, M.N.; Celik, A.; Yilmaz, M.; Kalayci, M.; Sahin, I.; Gungor, O.; Gurel, A.; et al. Cardiac, skeletal muscle and serum irisin responses to with or without water exercise in young and old male rats: Cardiac muscle produces more irisin than skeletal muscle. Peptides 2014, 52, 68–73. [Google Scholar] [CrossRef]
- MacKenzie, M.G.; Hamilton, D.L.; Pepin, M.; Patton, A.; Baar, K. Inhibition of Myostatin Signaling through Notch Activation following Acute Resistance Exercise. PLoS ONE 2013, 8, e68743. [Google Scholar] [CrossRef]
- Bergman, B.C.; Brozinick, J.T.; Strauss, A.; Bacon, S.; Kerege, A.; Bui, H.H.; Sanders, P.; Siddall, P.; Wei, T.; Thomas, M.K.; et al. Muscle sphingolipids during rest and exercise: A C18:0 signature for insulin resistance in humans. Diabetologia 2016, 59, 785–798. [Google Scholar] [CrossRef]
- Baranowski, M.; Błachnio-Zabielska, A.U.; Charmas, M.; Helge, J.W.; Dela, F.; Książek, M.; Długołęcka, B.; Klusiewicz, A.; Chabowski, A.; Górski, J. Exercise increases sphingoid base-1-phosphate levels in human blood and skeletal muscle in a time- and intensity-dependent manner. Eur. J. Appl. Physiol. 2014, 115, 993–1003. [Google Scholar] [CrossRef]
- Saba, J.D.; de la Garza-Rodea, A.S. S1P lyase in skeletal muscle regeneration and satellite cell activation: Exposing the hidden lyase. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2013, 1831, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.; Berezin, A.A.; Lichtenauer, M. Myokines and Heart Failure: Challenging Role in Adverse Cardiac Remodeling, Myopathy, and Clinical Outcomes. Dis. Markers 2021, 2021, 6644631. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.C.; Leong, W.-I.; Carlson, M.E.; Oskouian, B.; Kumar, A.; Fyrst, H.; Zhang, M.; Proia, R.L.; Hoffman, E.P.; Saba, J.D. Sphingosine-1-Phosphate Enhances Satellite Cell Activation in Dystrophic Muscles through a S1PR2/STAT3 Signaling Pathway. PLoS ONE 2012, 7, e37218. [Google Scholar] [CrossRef]
- Germinario, E.; Bondì, M.; Blaauw, B.; Betto, R.; Danieli-Betto, D. Reduction of circulating sphingosine-1-phosphate worsens mdx soleus muscle dystrophic phenotype. Exp. Physiol. 2020, 105, 1895–1906. [Google Scholar] [CrossRef]
- Meacci, E.; Pierucci, F.; Garcia-Gil, M. Skeletal Muscle and COVID-19: The Potential Involvement of Bioactive Sphingolipids. Biomedicines 2022, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Dozio, E.; Passeri, E.; Cardani, R.; Benedini, S.; Aresta, C.; Valaperta, R.; Romanelli, M.C.; Meola, G.; Sansone, V.; Corbetta, S. Circulating Irisin Is Reduced in Male Patients with Type 1 and Type 2 Myotonic Dystrophies. Front. Endocrinol. 2017, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jun, H.-S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo Transcription Factors Induce the Atrophy-Related Ubiquitin Ligase Atrogin-1 and Cause Skeletal Muscle Atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef]
- Chakkour, M.; Kreyddiyeh, S. FTY720P Upregulates the Na+/K+ ATPase in HepG2 Cells by Activating S1PR3 and Inducing PGE2 Release. Cell. Physiol. Biochem. 2019, 53, 518–531. [Google Scholar] [CrossRef]
- Gatta, L.; Vitiello, L.; Gorini, S.; Chiandotto, S.; Costelli, P.; Giammarioli, A.M.; Malorni, W.; Rosano, G.; Ferraro, E. Modulating the metabolism by trimetazidine enhances myoblast differentiation and promotes myogenesis in cachectic tumor-bearing c26 mice. Oncotarget 2017, 8, 113938–113956. [Google Scholar] [CrossRef]
- Efe, T.H.; Açar, B.; Ertem, A.G.; Yayla, K.G.; Algül, E.; Yayla, Ç.; Ünal, S.; Bilgin, M.; Çimen, T.; Kirbaş, Ö.; et al. Serum Irisin Level Can Predict the Severity of Coronary Artery Disease in Patients with Stable Angina. Korean Circ. J. 2017, 47, 44. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, F.; Frati, A.; Squecco, R.; Lenci, E.; Vicenti, C.; Slavik, J.; Francini, F.; Machala, M.; Meacci, E. Non-dioxin-like organic toxicant PCB153 modulates sphingolipid metabolism in liver progenitor cells: Its role in Cx43-formed gap junction impairment. Arch. Toxicol. 2017, 91, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, V.A.; Watson, C.J.F.; Bott, K.N.; Moradi, F.; Maddalena, L.A.; Bellissimo, C.A.; Turner, K.D.; Peters, S.J.; LeBlanc, P.J.; MacNeil, A.J.; et al. Neurogranin is expressed in mammalian skeletal muscle and inhibits calcineurin signaling and myoblast fusion. Am. J. Physiol. Physiol. 2019, 317, C1025–C1033. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Pini, A.; Chellini, F.; Mazzanti, B.; Nistri, S.; Nosi, D.; Saccardi, R.; Quercioli, F.; Zecchi-Orlandini, S.; Formigli, L. Bone Marrow Mesenchymal Stromal Cells Stimulate Skeletal Myoblast Proliferation through the Paracrine Release of VEGF. PLoS ONE 2012, 7, e37512. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierucci, F.; Chirco, A.; Meacci, E. Irisin Is Target of Sphingosine-1-Phosphate/Sphingosine-1-Phosphate Receptor-Mediated Signaling in Skeletal Muscle Cells. Int. J. Mol. Sci. 2023, 24, 10548. https://doi.org/10.3390/ijms241310548
Pierucci F, Chirco A, Meacci E. Irisin Is Target of Sphingosine-1-Phosphate/Sphingosine-1-Phosphate Receptor-Mediated Signaling in Skeletal Muscle Cells. International Journal of Molecular Sciences. 2023; 24(13):10548. https://doi.org/10.3390/ijms241310548
Chicago/Turabian StylePierucci, Federica, Antony Chirco, and Elisabetta Meacci. 2023. "Irisin Is Target of Sphingosine-1-Phosphate/Sphingosine-1-Phosphate Receptor-Mediated Signaling in Skeletal Muscle Cells" International Journal of Molecular Sciences 24, no. 13: 10548. https://doi.org/10.3390/ijms241310548