Adaptive Responses of a Peroxidase-like Polyoxometalate-Based Tri-Assembly to Bacterial Microenvironment (BME) Significantly Improved the Anti-Bacterial Effects
Abstract
:1. Introduction
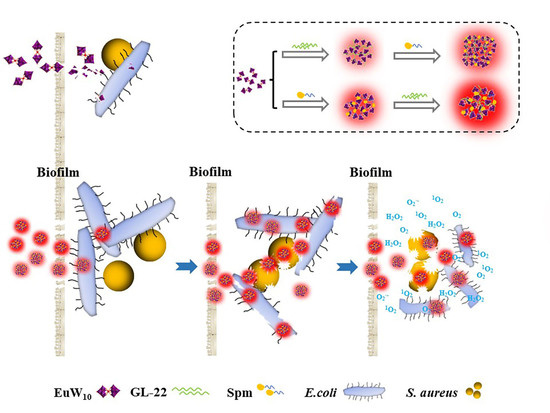
2. Results
2.1. Construction and Characterizations of the Tertiary Assembly of EuW10/Spm/GL-22
2.2. Enhanced Antibacterial Effects of the Bi- and Tri-Assembly
2.3. Dose-Dependent Antibacterial Effects of the Constituent in Tri-Assemblies
3. Discussion
3.1. Intrinsic Mechanism behind the Enhanced Antibacterial Effects of Tri-Assembly
3.1.1. The Peroxidase-like Activity of EuW10
3.1.2. The Enhanced ROS Generation of Tri-Assembly over EuW10 in E. coli
3.2. Assembly Promoted EuW10 Uptake
3.3. Enhanced Biofilm Elimination of Assemblies
3.4. Correlation between Particle Size of the Assembly and Antibacterial Effect
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Equipment and Characterization
4.3. Construction of the Assemblies
4.3.1. Construction of Bi-Assemblies of EuW10/GL-22 and EuW10/Spm
4.3.2. Construction of Tri-Assemblies of EuW10/Spm/GL-22 and EuW10/GL-22/Spm
4.4. Bacteria Culture
4.5. Anti-Bacteria Assay via Agar Plates
4.6. Anti-Bacteria Assay Based on OD600
4.7. Reactive Oxygen Species (ROS) Assay
4.8. Biofilm Elimination Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, P.A.; Koehler, M.F.T.; Girgis, H.S.; Yan, D.H.; Chen, Y.S.; Chen, Y.; Crawford, J.J.; Durk, M.R.; Higuchi, R.I.; Kang, J.; et al. Optimized arylomycins are a new class of Gram-negative antibiotics. Nature 2018, 561, 189–194. [Google Scholar] [CrossRef]
- Boehle, K.E.; Gilliand, J.; Wheeldon, C.R.; Holder, A.; Adkins, J.A.; Geiss, B.J.; Ryan, E.P.; Henry, C.S. Utilizing paper-based devices for antimicrobial-resistant bacteria detection. Angew. Chem. Int. Ed. 2017, 56, 6886–6890. [Google Scholar] [CrossRef]
- Krol, A.; Pomastowski, P.; Rafinska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Xie, Y.-Y.; Hu, X.-H.; Zhang, Y.-W.; Wahid, F.; Chu, L.-Q.; Jia, S.-R.; Zhong, C. Development and antibacterial activities of bacterial cellulose/graphene oxide-CuO nanocomposite films. Carbohydr. Polym. 2020, 229, 115456. [Google Scholar] [CrossRef]
- Daima, H.K.; Selvakannan, P.R.; Shukla, R.; Bhargava, S.K.; Bansal, V. Fine-tuning the antimicrobial profile of biocompatible gold nanoparticles by sequential surface functionalization using polyoxometalates and lysine. PLoS ONE 2013, 8, e79676. [Google Scholar] [CrossRef]
- Fu, D.-Y.; Zhang, S.; Qu, Z.; Yu, X.; Wu, Y.; Wu, L. Hybrid assembly toward enhanced thermal stability of virus-like particles and antibacterial activity of polyoxometalates. ACS Appl. Mater. Inter. 2018, 10, 6137–6145. [Google Scholar] [CrossRef]
- Daima, H.K.; Selvakannan, P.R.; Kandjani, A.E.; Shukla, R.; Bhargava, S.K.; Bansal, V. Synergistic influence of polyoxometalate surface corona towards enhancing the antibacterial performance of tyrosine-capped Ag nanoparticles. Nanoscale 2014, 6, 758–765. [Google Scholar] [CrossRef]
- Kong, X.; Wan, G.; Li, B.; Wu, L. Recent advances of polyoxometalates in multi-functional imaging and photothermal therapy. J. Mater. Chem. B 2020, 8, 8189–8206. [Google Scholar] [CrossRef]
- Casan-Pastor, N.; Gomez-Romero, P. Polyoxometalates: From inorganic chemistry to materials science. Front. Biosci. Landmark 2004, 9, 1759–1770. [Google Scholar] [CrossRef]
- Prashant, V.K.; Masakazu, A. Environmentally Benign Catalysts; Springer: Dordrecht, The Netherlands, 2013; pp. 245–255. [Google Scholar]
- Bijelic, A.; Rompel, A. Ten good reasons for the use of the tellurium-centered Anderson-evans polyoxotungstate in protein crystallography. Acc. Chem. Res. 2017, 50, 1441–1448. [Google Scholar] [CrossRef]
- Wang, S.-S.; Yang, G.-Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef]
- Rhule, J.T.; Hill, C.L.; Judd, D.A.; Schinazi, R.F. Polyoxometalates in Medicine. Chem. Rev. 1998, 98, 327–358. [Google Scholar] [CrossRef]
- Arefian, M.; Mirzaei, M.; Eshtiagh-Hosseini, H.; Frontera, A. A survey of the different roles of polyoxometalates in their interaction with amino acids, peptides and proteins. Dalton Trans. 2017, 46, 6812–6829. [Google Scholar] [CrossRef]
- Yamase, T. Anti-tumor, -viral, and -bacterial activities of polyoxometalates for realizing an inorganic drug. J. Mater. Chem. 2005, 15, 4773–4782. [Google Scholar] [CrossRef]
- Chi, G.; Wang, L.; Chen, B.; Li, J.; Hu, J.; Liu, S.; Zhao, M.; Ding, X.; Li, Y. Polyoxometalates: Study of inhibitory kinetics and mechanism against a-glucosidase. J. Inorg. Biochem. 2019, 199, 110784. [Google Scholar] [CrossRef]
- Bijelic, A.; Aureliano, M.; Rompel, A. Polyoxometalates as potential next-generation metallodrugs in the combat against cancer. Angew. Chem. Int. Ed. 2019, 58, 2980–2999. [Google Scholar] [CrossRef]
- Judd, D.A.; Nettles, J.H.; Nevins, N.; Snyder, J.P.; Liotta, D.C.; Tang, J.; Ermolieff, J.; Schinazi, R.F.; Hill, C.L. Polyoxometalate HIV–1 protease inhibitors. A new mode of protease inhibition. J. Am. Chem. Soc. 2001, 123, 886–897. [Google Scholar] [CrossRef]
- Molitor, C.; Bijelic, A.; Rompel, A. In situ formation of the first proteinogenically functionalized [TeW6O24O2(Glu)]7– structure reveals unprecedented chemical and geometrical features of the Anderson-type cluster. Chem. Commun. 2016, 52, 12286–12289. [Google Scholar] [CrossRef]
- Molitor, C.; Bijelic, A.; Rompel, A. The potential of hexatungstotellurate (VI) to induce a significant entropic gain during protein crystallization. IUCrJ 2017, 4, 734–740. [Google Scholar] [CrossRef]
- Bijelic, A.; Aureliano, M.; Rompel, A. The antibacterial activity of polyoxometalates: Structures, antibiotic effects and future perspectives. Chem. Commun. 2018, 54, 1153–1169. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Zhou, M.; Jing, J.; Li, W.; Wang, Y.; Wu, L.; Wang, L.; Wang, Y.; Lee, M. Polyoxometalate-driven self-assembly of short peptides into multivalent nanofibers with enhanced antibacterial activity. Angew. Chem. Int. Ed. 2016, 55, 2592–2595. [Google Scholar] [CrossRef]
- Dahms, S.O.; Kuester, M.; Streb, C.; Roth, C.; Straeter, N.; Than, M.E. Localization and orientation of heavy-atom cluster compounds in protein crystals using molecular replacement. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 284–297. [Google Scholar] [CrossRef]
- Griffith, W.P.; Morley-Smith, N.; Nogueira, H.I.S.; Shoair, A.G.F.; Suriaatmaja, M.; White, A.J.P.; Williams, D.J. Studies on polyoxo and polyperoxo-metalates Part 7. Lanthano- and thoriopolyoxotungstates as catalytic oxidants with H2O2 and the X-ray crystal structure of Na8[ThW10O36]·28H2O. J. Organomet. Chem. 2000, 607, 146–155. [Google Scholar] [CrossRef]
- Zhang, T.; Li, H.-W.; Wu, Y.; Wang, Y.; Wu, L. Self-assembly of an europium-containing polyoxometalate and the arginine/lysine-rich peptides from human papillomavirus capsid protein L1 in forming luminescence-enhanced hybrid nanoparticles. J. Phys. Chem. C 2015, 119, 8321–8328. [Google Scholar] [CrossRef]
- Zhang, C.-X.; Kong, X.; Li, H.-W.; Li, B.; Wu, L.; Wu, Y. A sustainable luminescence-enhanced tri-assembly of polyoxometalate-peptide-polyamine developed for ultrasensitive spermine determination and discrimination. Colloids Surface B 2022, 212, 112379. [Google Scholar] [CrossRef]
- Kaper, J.B.; Mills, A.L.; Colwell, R.R. Evaluation of the accuracy and precision of enumerating aerobic heterotrophs in water samples by the spread plate method. Appl. Environ. Microbiol. 1978, 35, 756–761. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Al-Sayed, E.; Krivosudsky, L.; Cipic-Paljetak, H.; Verbanac, D.; Rompel, A. Antibacterial activity of polyoxometalates against moraxella catarrhalis. Front. Chem. 2018, 6, 336. [Google Scholar] [CrossRef]
- Taleb, H.; Maddocks, S.E.; Morris, R.K.; Kanekanian, A.D. The antibacterial activity of date syrup polyphenols against S. aureus and E. coli. Front. Microbiol. 2016, 7, 198. [Google Scholar] [CrossRef]
- Wu, C.-L.; Hsueh, J.-Y.; Yip, B.-S.; Chih, Y.-H.; Peng, K.-L.; Cheng, J.-W. Antimicrobial peptides display strong synergy with vancomycin against vancomycin-resistant E. faecium, S. aureus, and wild-type E. coli. Int. J. Mol. Sci. 2020, 21, 4578. [Google Scholar] [CrossRef]
- Diaz, R.; Ramalheira, E.; Afreixo, V.; Gago, B. Evaluation of vancomycin MIC creep in Staphylococcus aureus. J. Glob. Antimicrob. Resist. 2017, 10, 281–284. [Google Scholar] [CrossRef]
- Zhang, H.L.; Zhao, Y.Y.; Zhou, Z.C.; Ding, H.Z. Susceptibility breakpoint for cefquinome against Escherichia coli and Staphylococcus aureus from pigs. J. Integr. Agric. 2021, 20, 1921–1932. [Google Scholar] [CrossRef]
- Nazari, S.; Gholami, M.; Farzadkia, M.; Dourbash, F.A.; Arzanlou, M.; Kalantary, R.R. Synthesis and evaluation of the antibacterial effect of silica-coated modified magnetic poly-(amidoamine) G5 nanoparticles on E. coli and S. aureus. J. Mol. Liq. 2019, 276, 93–104. [Google Scholar] [CrossRef]
- Yang, F.-C.; Wu, K.-H.; Lin, W.-P.; Hu, M.-K. Preparation and antibacterial efficacy of bamboo charcoal/polyoxometalate biological protective material. Microporous Mesoporous Mater. 2009, 118, 467–472. [Google Scholar] [CrossRef]
- Joshi, G.S.; Spontak, J.S.; Klapper, D.G.; Richardson, A.R. Arginine catabolic mobile element encoded speG abrogates the unique hypersensitivity of Staphylococcus aureus to exogenous polyamines. Mol. Microbiol. 2011, 82, 9–20. [Google Scholar] [CrossRef]
- Muppidi, A.; Li, X.; Chen, J.; Qing, L. Conjugation of spermine enhances cellular uptake of the stapled peptide-based inhibitors of p53-Mdm2 interaction. Bioorg. Med. Chem. Lett. 2011, 21, 7412–7415. [Google Scholar] [CrossRef]
- Hou, Z.; Shankar, Y.V.; Liu, Y.; Ding, F.; Subramanion, J.L.; Ravikumar, V.; Zamudio-Vazquez, R.; Keogh, D.; Lim, H.; Tay, M.Y.F.; et al. Nanoparticles of short cationic peptidopolysaccharide self-assembled by hydrogen bonding with antibacterial effect against multidrug-resistant bacteria. ACS Appl. Mater. Interfaces 2017, 9, 38288–38303. [Google Scholar] [CrossRef]
- He, B.; Ma, S.; Peng, G.; He, D. TAT-modified self-assembled cationic peptide nanoparticles as an efficient antibacterial agent. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 365–372. [Google Scholar] [CrossRef]
- Panduwawala, T.D.; Iqbal, S.; Thompson, A.L.; Genov, M.; Pretsch, A.; Pretsch, D.; Liu, S.; Ebright, R.H.; Howells, A.; Maxwell, A.; et al. Functionalised bicyclic tetramates derived from cysteine as antibacterial agents. Org. Biomol. Chem. 2019, 17, 5615–5632. [Google Scholar] [CrossRef]
- Herzog, I.M.; Fridman, M. Design and synthesis of membrane-targeting antibiotics: From peptides- to aminosugar-based antimicrobial cationic amphiphiles. MedChemComm 2014, 5, 1014–1026. [Google Scholar] [CrossRef]
- Moradlou, O.; Rabiei, Z.; Delavari, N. Antibacterial effects of carbon quantum dots@hematite nanostructures deposited on titanium against Gram-positive and Gram-negative bacteria. J. Photochem. Photobiol. A Chem. 2019, 379, 144–149. [Google Scholar] [CrossRef]
- Botar, B.; Ellern, A.; Hermann, R.; Kögerler, P. Electronic control of spin coupling in keplerate-type polyoxomolybdates. Angew. Chem. Int. Ed. 2009, 48, 9080–9083. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, L.-Y.; Jiao, J.; Xin, X.; Sun, D.; Yuan, S. Ionic self-assembly of polyoxometalate dopamine hybrid nanoflowers with excellent catalytic activity for dyes. ACS Sustain. Chem. Eng. 2017, 5, 1358–1367. [Google Scholar] [CrossRef]
- Afri, M.; Frimer, A.A.; Cohen, Y. Active oxygen chemistry within the liposomal bilayer: Part IV: Locating 2′,7′-dichlorofluorescein (DCF), 2′,7′-dichlorodihydrofluorescein (DCFH) and 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) in the lipid bilayer. Chem. Phys. Lipids 2004, 131, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liang, J.; Xiao, G.; Vargas-De-La-Cruz, C.; Simal-Gandara, J.; Xiao, J.; Wang, Q. Active sites of peptides Asp-Asp-Asp-Tyr and Asp-Tyr-Asp-Asp protect against cellular oxidative stress. Food Chem. 2022, 366, 130626. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Zhou, M.-L.; Johnson, A.W.; Singh, I.; Liao, F.; Vellimana, A.K.; Nelso, J.W.; Milner, E.; Cirrito, J.R.; Basak, J.; et al. Contribution of reactive oxygen species to cerebral amyloid angiopathy, vasomotor dysfunction, and microhemorrhage in aged Tg2576 mice. Proc. Natl. Acad. Sci. USA 2015, 112, E881–E890. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ran, X.; Lin, Y.; Ren, J.; Qu, X. Self-assembly of an organic-inorganic hybrid nanoflower as an efficient biomimetic catalyst for self-activated tandem reactions. Chem. Commun. 2015, 51, 4386–4389. [Google Scholar] [CrossRef] [PubMed]
- Kitatsuji, C.; Izumi, K.; Nambu, S.; Kurogochi, M.; Uchida, T.; Nishimura, S.-I.; Iwai, K.R.; O’Brian, M.; Ikeda-Saito, M.; Ishimori, K. Protein oxidation mediated by heme-induced active site conversion specific for heme-regulated transcription factor, iron response regulator. Sci. Rep. 2016, 6, 18703. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lu, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Simoes, M.; Bennett, R.N.; Rosa, E.A.S. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat. Prod. Rep. 2009, 26, 746–757. [Google Scholar] [CrossRef]
- Li, X.; Wu, B.; Chen, H.; Nan, K.H.; Jin, Y.Y.; Sun, L.; Wang, B.L. Recent developments in smart antibacterial surfaces to inhibit biofilm formation and bacterial infections. J. Mater. Chem. B 2018, 6, 4274–4292. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, K.; Liu, Z.; Zhang, Y.; Chen, Z.; Sun, H.; Ren, J.; Qu, X. Activation of biologically relevant levels of reactive oxygen species by Au/g-C3N4 hybrid nanozyme for bacteria killing and wound disinfection. Biomaterials 2017, 113, 145–157. [Google Scholar] [CrossRef]
- Agrawal, N.; Mishra, P.; Ranjan, R.; Awasthi, P.; Srivastava, A.; Prasad, D.; Kohli, E. Nano-cubes over nano-spheres: Shape dependent study of silver nanomaterial for biological applications. Bull. Mater. Sci. 2021, 44, 191. [Google Scholar] [CrossRef]
- Goyal, S.; Gupta, N.; Kumar, A.; Chatterjee, S.; Nimesh, S. Antibacterial, anticancer and antioxidant potential of silver nanoparticles engineered using Trigonella foenum-graecum seed extract. IET Nanobiotechnol. 2018, 12, 526–533. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Liu, R.; Kong, X.; Li, H.; Yu, D.; Fang, X.; Wu, L.; Wu, Y. Adaptive Responses of a Peroxidase-like Polyoxometalate-Based Tri-Assembly to Bacterial Microenvironment (BME) Significantly Improved the Anti-Bacterial Effects. Int. J. Mol. Sci. 2023, 24, 8858. https://doi.org/10.3390/ijms24108858
Zhang C, Liu R, Kong X, Li H, Yu D, Fang X, Wu L, Wu Y. Adaptive Responses of a Peroxidase-like Polyoxometalate-Based Tri-Assembly to Bacterial Microenvironment (BME) Significantly Improved the Anti-Bacterial Effects. International Journal of Molecular Sciences. 2023; 24(10):8858. https://doi.org/10.3390/ijms24108858
Chicago/Turabian StyleZhang, Chunxia, Rongrong Liu, Xueping Kong, Hongwei Li, Dahai Yu, Xuexun Fang, Lixin Wu, and Yuqing Wu. 2023. "Adaptive Responses of a Peroxidase-like Polyoxometalate-Based Tri-Assembly to Bacterial Microenvironment (BME) Significantly Improved the Anti-Bacterial Effects" International Journal of Molecular Sciences 24, no. 10: 8858. https://doi.org/10.3390/ijms24108858