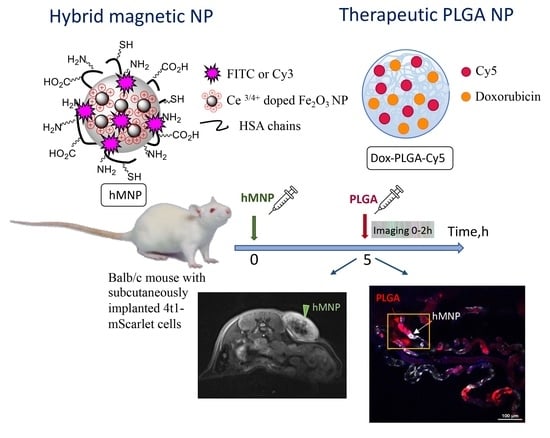
Supermagnetic Human Serum Albumin (HSA) Nanoparticles and PLGA-Based Doxorubicin Nanoformulation: A Duet for Selective Nanotherapy
Abstract
:1. Introduction
2. Results
2.1. Nanoparticle Preparation and Characterization
2.2. Optical Properties of Nanoparticles
2.3. Investigation of PLGA NP and hMNP Internalization into 4T1 Cells In Vitro
2.3.1. Internalization of Dox-PLGA-Cy5 Nanoparticles
2.3.2. Internalization of PLGA-Cy5 NP and hMNP-Cy3 into 4T1 Cells upon Co-Incubation
2.3.3. Investigation of the PLGA-Cy5 NP and hMNP Internalization Pathway in 4T1 mScarlet Cells Using Endocytosis Inhibitors
2.4. Investigation of hMNP In Vivo Distribution in the 4T1 Tumor Using MRI
2.5. Investigation of the Nanoparticle Distribution in Mice Bearing Subcutaneous 4T1 Tumor Using Intravital Fluorescence Microscopy
2.5.1. Evaluation of Blood Circulation Half-Lives
2.5.2. Investigation of the Nanoparticle Microdistribution in Tumor Vasculature and Peritumoral Area in 4T1-mScarlet Tumor-Bearing Mice upon Simultaneous Administration
2.5.3. Investigation of hMNP and PLGA NP Microdistribution in Tumor Vasculature and Peritumoral Area in 4T1-mScarlet Tumor-Bearing Mice upon Different Treatment Regimens
2.5.4. Quantitative Evaluation of the Prognostic Potential of the Theranostic Pair
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Fluorescently Labeled Hybrid Ce3/4+-Doped Maghemite NPs Encapsulated in HSA Matrix (hMNP)
4.3. Preparation of Doxorubicin-Loaded PLGA Nanoparticles Labeled with Cyanine5 (Dox-PLGA-Cy5)
4.4. Nanoparticle Characterization
4.4.1. Size, Polydispersity Index (PDI) and Zeta-Potential
4.4.2. Dox-PLGA-Cy5 Nanoparticles: Evaluation of Drug Loading, Encapsulation Efficiency, and Drug Release Rate
4.4.3. Drug Release Studies
4.4.4. Quantitative Analysis of Elemental Composition of the hMNP
4.4.5. Fluorescence Spectroscopy
4.4.6. Scanning Electron Microscopy (SEM) of PLGA NP
4.4.7. Transmission Electron Microscopy (TEM) of hMNP and PLGA NP
4.5. In Vitro Experiments
4.5.1. 4T1 Murine Mammary Carcinoma Cells
4.5.2. Investigation of Nanoparticle Internalization by 4T1 Tumor Cells In Vitro
4.5.3. Evaluation of hMNP Hemocompatibility and Cytotoxicity
4.6. In Vivo Experiments
4.6.1. Tumor Models
4.6.2. Determination of T2 Relaxivities for Magnetic Nanoparticles
4.6.3. Magnetic Resonance Imaging
4.6.4. Intravital Microscopy
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the Dynamics of the EPR Effect and Strategies to Improve the Therapeutic Effects of Nanomedicines by Using EPR Effect Enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Toward a Full Understanding of the EPR Effect in Primary and Metastatic Tumors as Well as Issues Related to Its Heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor Targeting via EPR: Strategies to Enhance Patient Responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Danhier, F. To Exploit the Tumor Microenvironment: Since the EPR Effect Fails in the Clinic, What Is the Future of Nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Grodzinski, P.; Kircher, M.; Goldberg, M.; Gabizon, A. Integrating Nanotechnology into Cancer Care. ACS Nano 2019, 13, 7370–7376. [Google Scholar] [CrossRef] [Green Version]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [Green Version]
- Ramón Cajal, S.; Sesé, M.; Capdevila, C.; Aasen, T.; Mattos-Arruda, L.; Diaz-Cano, S.J.; Hernández-Losa, J.; Castellví, J. Clinical Implications of Intratumor Heterogeneity: Challenges and Opportunities. J. Mol. Med. 2020, 98, 161–177. [Google Scholar] [CrossRef] [Green Version]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Gregory Roberts, W.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of Transport Pathways in Tumor Vessels: Role of Tumor Type and Microenvironment. Med. Sci. 1998, 95, 4607–4612. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.K. Transport of Molecules, Particles, and Cells in Solid Tumors. Annu. Rev. Biomed. Eng. 1999, 1, 241–263. [Google Scholar] [CrossRef]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between Defective Endothelial Cells Explain Tumor Vessel Leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Q.; Fathi, P.; Chen, X. Nanoparticle Delivery in Vivo: A Fresh Look from Intravital Imaging. EBioMedicine 2020, 59, 102958. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Nichols, J.W.; Toh, K.; Nomoto, T.; Cabral, H.; Miura, Y.; Christie, R.J.; Yamada, N.; Ogura, T.; Kano, M.R.; et al. Vascular Bursts Enhance Permeability of Tumour Blood Vessels and Improve Nanoparticle Delivery. Nat. Nanotechnol. 2016, 11, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The Entry of Nanoparticles into Solid Tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- Naumenko, V.A.; Vlasova, K.Y.; Garanina, A.S.; Melnikov, P.A.; Potashnikova, D.M.; Vishnevskiy, D.A.; Vodopyanov, S.S.; Chekhonin, V.P.; Abakumov, M.A.; Majouga, A.G. Extravasating Neutrophils Open Vascular Barrier and Improve Liposomes Delivery to Tumors. ACS Nano 2019, 13, 12599–12612. [Google Scholar] [CrossRef]
- Wu, J. Personalized Medicine The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Miller, M.A.; Gadde, S.; Pfirschke, C.; Engblom, C.; Sprachman, M.M.; Kohler, R.H.; Yang, K.S.; Laughney, A.M.; Wojtkiewicz, G.; Kamaly, N.; et al. Predicting Therapeutic Nanomedicine Efficacy Using a Companion Magnetic Resonance Imaging Nanoparticle. Sci. Transl. Med. 2015, 7, 314ra183. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.A.; Arlauckas, S.; Weissleder, R. Prediction of Anti-Cancer Nanotherapy Efficacy by Imaging. Nanotheranostics 2017, 1, 296–312. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.; Bridot, J.L.; Elst, L.V.; Muller, R.N. Magnetic Iron Oxide Nanoparticles for Biomedical Applications. Future Med. Chem. 2010, 2, 427–449. [Google Scholar] [CrossRef] [Green Version]
- Zhi, D.; Yang, T.; Yang, J.; Fu, S.; Zhang, S. Targeting Strategies for Superparamagnetic Iron Oxide Nanoparticles in Cancer Therapy. Acta Biomater. 2020, 102, 13–34. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Gorgoń, S.; Radoń, A.; Bajdak-Rusinek, K. Magnetite Nanoparticles in Magnetic Hyperthermia and Cancer Therapies: Challenges and Perspectives. Nanomaterials 2022, 12, 1807. [Google Scholar] [CrossRef] [PubMed]
- Lemine, O.M.; Madkhali, N.; Alshammari, M.; Algessair, S.; Gismelseed, A.; Mir, L.E.; Hjiri, M.; Yousif, A.A.; El-Boubbou, K. Maghemite (γ-Fe2O3) and γ-Fe2O3-TiO2 Nanoparticles for Magnetic Hyperthermia Applications: Synthesis, Characterization and Heating Efficiency. Materials 2021, 14, 5691. [Google Scholar] [CrossRef] [PubMed]
- Fortuin, A.S.; Brüggemann, R.; Linden, J.; Panfilov, I.; Israël, B.; Scheenen, T.W.J.; Barentsz, J.O. Ultra-small Superparamagnetic Iron Oxides for Metastatic Lymph Node Detection: Back on the Block. WIREs Nanomed. Nanobiotechnology 2018, 10, e1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Kumar, R.; Nagesha, D.; Duclos, R.I.; Sridhar, S.; Gatley, S.J. Integrity of 111In-Radiolabeled Superparamagnetic Iron Oxide Nanoparticles in the Mouse. Nucl. Med. Biol. 2015, 42, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Elhalawani, H.; Awan, M.J.; Ding, Y.; Mohamed, A.S.R.; Elsayes, A.K.; Abu-Gheida, I.; Wang, J.; Hazle, J.; Gunn, G.B.; Lai, S.Y.; et al. Data from a Terminated Study on Iron Oxide Nanoparticle Magnetic Resonance Imaging for Head and Neck Tumors. Sci. Data 2020, 7, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israel, L.L.; Lellouche, E.; Kenett, R.S.; Green, O.; Michaeli, S.; Lellouche, J.-P. Ce3/4+ Cation-Functionalized Maghemite Nanoparticles towards SiRNA-Mediated Gene Silencing. J. Mater. Chem. B 2014, 2, 6215–6225. [Google Scholar] [CrossRef]
- Haimov-Talmoud, E.; Harel, Y.; Schori, H.; Motiei, M.; Atkins, A.; Popovtzer, R.; Lellouche, J.-P.; Shefi, O. Magnetic Targeting of MTHPC To Improve the Selectivity and Efficiency of Photodynamic Therapy. ACS App.l Mater. Interfaces 2019, 11, 45368–45380. [Google Scholar] [CrossRef] [PubMed]
- Ishay, R.B.; Israel, L.L.; Eitan, E.L.; Partouche, D.M.; Lellouche, J.-P. Maghemite-Human Serum Albumin Hybrid Nanoparticles: Towards a Theranostic System with High MRI r2* Relaxivity. J. Mater. Chem. B 2016, 4, 3801–3814. [Google Scholar] [CrossRef]
- Gossuin, Y.; Martin, E.; Vuong, Q.L.; Delroisse, J.; Laurent, S.; Stanicki, D.; Rousseau, C. Characterization of Commercial Iron Oxide Clusters with High Transverse Relaxivity. J. Magn. Reson. Open 2022, 10–11, 100054. [Google Scholar] [CrossRef]
- Khramtsov, P.; Barkina, I.; Kropaneva, M.; Bochkova, M.; Timganova, V.; Nechaev, A.; Byzov, I.; Zamorina, S.; Yermakov, A.; Rayev, M. Magnetic Nanoclusters Coated with Albumin, Casein, and Gelatin: Size Tuning, Relaxivity, Stability, Protein Corona, and Application in Nuclear Magnetic Resonance Immunoassay. Nanomaterials 2019, 9, 1345. [Google Scholar] [CrossRef]
- Sakulkhu, U.; Mahmoudi, M.; Maurizi, L.; Salaklang, J.; Hofmann, H. Protein Corona Composition of Superparamagnetic Iron Oxide Nanoparticles with Various Physico-Chemical Properties and Coatings. Sci. Rep. 2014, 4, 5020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velichko, E.N.; Nepomnyashchaya, E.K.; Baranov, M.A.; Skvortsov, A.N.; Pleshakov, I.V.; Dong, G. Aggregation Properties of Albumin in Interacting with Magnetic Fluids. Int. J. Mol. Sci. 2021, 22, 10734. [Google Scholar] [CrossRef] [PubMed]
- Chubarov, A.S. Serum Albumin for Magnetic Nanoparticles Coating. Magnetochemistry 2022, 8, 13. [Google Scholar] [CrossRef]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef]
- Cho, H.; Jeon, S.I.; Ahn, C.-H.; Shim, M.K.; Kim, K. Emerging Albumin-Binding Anticancer Drugs for Tumor-Targeted Drug Delivery: Current Understandings and Clinical Translation. Pharmaceutics 2022, 14, 728. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, A.L.; Pangua, C.; Reboredo, C.; Campión, R.; Morales-Gracia, J.; Irache, J.M. Protein-Based Nanoparticles for Drug Delivery Purposes. Int. J. Pharm. 2020, 581, 119289. [Google Scholar] [CrossRef]
- Baki, A.; Remmo, A.; Löwa, N.; Wiekhorst, F.; Bleul, R. Albumin-Coated Single-Core Iron Oxide Nanoparticles for Enhanced Molecular Magnetic Imaging (MRI/MPI). Int. J. Mol. Sci. 2021, 22, 6235. [Google Scholar] [CrossRef]
- Moya, C.; Escudero, R.; Malaspina, D.C.; de la Mata, M.; Hernández-Saz, J.; Faraudo, J.; Roig, A. Insights into Preformed Human Serum Albumin Corona on Iron Oxide Nanoparticles: Structure, Effect of Particle Size, Impact on MRI Efficiency, and Metabolization. ACS Appl. Bio. Mater. 2019, 2, 3084–3094. [Google Scholar] [CrossRef]
- Maksimenko, O.; Malinovskaya, J.; Shipulo, E.; Osipova, N.; Razzhivina, V.; Arantseva, D.; Yarovaya, O.; Mostovaya, U.; Khalansky, A.; Fedoseeva, V.; et al. Doxorubicin-loaded PLGA nanoparticles for the chemotherapy of glioblastoma: Towards the pharmaceutical development. Int. J. Pharm. 2019, 15, 118733. [Google Scholar] [CrossRef]
- Filon, O.; Krivorotko, P.; Kobyakov, G.; Razjivina, V.; Maximenko, O.; Gelperina, S.; Kreuter, J. A Phase I Study of Safety and Pharmacokinetics of NanoBB-1-Dox in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2017, 35, e13537. [Google Scholar] [CrossRef]
- Pereverzeva, E.; Treschalin, I.; Treschalin, M.; Arantseva, D.; Ermolenko, Y.; Kumskova, N.; Maksimenko, O.; Balabanyan, V.; Kreuter, J.; Gelperina, S. Toxicological Study of Doxorubicin-Loaded PLGA Nanoparticles for the Treatment of Glioblastoma. Int. J. Pharm. 2019, 554, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Mei, K.C.; Bai, J.; Lorrio, S.; Wang, J.T.W.; Al-Jamal, K.T. Investigating the Effect of Tumor Vascularization on Magnetic Targeting in Vivo Using Retrospective Design of Experiment. Biomaterials 2016, 106, 276–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumenko, V.; Nikitin, A.; Garanina, A.; Melnikov, P.; Vodopyanov, S.; Kapitanova, K.; Potashnikova, D.; Vishnevskiy, D.; Alieva, I.; Ilyasov, A.; et al. Neutrophil-Mediated Transport Is Crucial for Delivery of Short-Circulating Magnetic Nanoparticles to Tumors. Acta Biomater. 2020, 104, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Malinovskaya, Y.; Melnikov, P.; Baklaushev, V.; Gabashvili, A.; Osipova, N.; Mantrov, S.; Ermolenko, Y.; Maksimenko, O.; Gorshkova, M.; Balabanyan, V.; et al. Delivery of Doxorubicin-Loaded PLGA Nanoparticles into U87 Human Glioblastoma Cells. Int. J. Pharm. 2017, 524, 77–90. [Google Scholar] [CrossRef]
- Zhukova, V.; Osipova, N.; Semyonkin, A.; Malinovskaya, J.; Melnikov, P.; Valikhov, M.; Porozov, Y.; Solovev, Y.; Kuliaev, P.; Zhang, E.; et al. Fluorescently Labeled PLGA Nanoparticles for Visualization In Vitro and In Vivo: The Importance of Dye Properties. Pharmaceutics 2021, 23, 1145. [Google Scholar] [CrossRef] [PubMed]
- Petri-Fink, A.; Hofmann, H. Superparamagnetic Iron Oxide Nanoparticles (SPIONs): From Synthesis to in Vivo Studies--a Summary of the Synthesis, Characterization, in Vitro, and in Vivo Investigations of SPIONs with Particular Focus on Surface and Colloidal Properties. IEEE Trans. Nanobiosci. 2007, 6, 289–297. [Google Scholar] [CrossRef]
- Israel, L.L.; Kovalenko, E.I.; Boyko, A.A.; Sapozhnikov, A.M.; Rosenberger, I.; Kreuter, J.; Passoni, L.; Lellouche, J.-P. Towards Hybrid Biocompatible Magnetic RHuman Serum Albumin-Based Nanoparticles: Use of Ultra-Small (CeLn)3/4+ Cation-Doped Maghemite Nanoparticles as Functional Shell. Nanotechnology 2015, 26, 045601. [Google Scholar] [CrossRef]
- Gonnissen, D.; Qu, Y.; Langer, K.; Öztürk, C.; Zhao, Y.; Chen, C.; Seebohm, G.; Dufer, M.; Fuchs, H.; Galla, H.-J.; et al. Comparison of Cellular Effects of Starch-Coated SPIONs and Poly(Lactic-Co-Glycolic Acid) Matrix Nanoparticles on Human Monocytes. Int. J. Nanomed. 2016, 11, 5221–5236. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, N.; Hillaireau, H.; Vergnaud, J.; Tsapis, N.; Pallardy, M.; Kerdine-Römer, S.; Fattal, E. Surface Coating Mediates the Toxicity of Polymeric Nanoparticles towards Human-like Macrophages. Int. J. Pharm. 2015, 482, 75–83. [Google Scholar] [CrossRef]
- Mohan, P.; Rapoport, N. Doxorubicin as a Molecular Nanotheranostic Agent: Effect of Doxorubicin Encapsulation in Micelles or Nanoemulsions on the Ultrasound-Mediated Intracellular Delivery and Nuclear Trafficking. Mol. Pharm. 2010, 7, 1959–1973. [Google Scholar] [CrossRef]
- Dunn, K.W.; Kamocka, M.M.; Mcdonald, J.H. A Practical Guide to Evaluating Colocalization in Biological Microscopy. Am. J. Physiol. Cell Physiol. 2011, 300, 723–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.H.; Rothberg, K.G.; Anderson, R.G. Mis-Assembly of Clathrin Lattices on Endosomes Reveals a Regulatory Switch for Coated Pit Formation. J. Cell Biol. 1993, 123, 1107–1117. [Google Scholar] [CrossRef]
- Rodal, S.K.; Skretting, G.; Garred, Ø.; Vilhardt, F.; van Deurs, B.; Sandvig, K. Extraction of Cholesterol with Methyl-Cyclodextrin Perturbs Formation of Clathrin-Coated Endocytic Vesicles. Mol. Biol. Cell 1999, 10, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Ben-Josef, E.; Robb, R.; Vedaie, M.; Seum, S.; Thirumoorthy, K.; Palanichamy, K.; Harbrecht, M.; Chakravarti, A.; Williams, T.M. Caveolae-Mediated Endocytosis Is Critical for Albumin Cellular Uptake and Response to Albumin-Bound Chemotherapy. Cancer Res. 2017, 77, 5925–5937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Y.; Zhou, Z.; Fan, M.; Gong, T.; Zhang, Z.; Sun, X. PEGylated Cationic Vectors Containing a Protease-Sensitive Peptide as a MiRNA Delivery System for Treating Breast Cancer. Mol. Pharm. 2017, 14, 81–92. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, L.; Su, H.; Zhou, D.; Song, H.; Wang, L.; Jiang, X. Poly(Ethylene Glycol)-Block-Poly(ε-Caprolactone)-and Phospholipid-Based Stealth Nanoparticles with Enhanced Therapeutic Efficacy on Murine Breast Cancer by Improved Intracellular Drug Delivery. Int. J. Nanomed. 2015, 10, 1791–1804. [Google Scholar] [CrossRef] [Green Version]
- Fornaguera, C.; Caldero, G.; Mitjans, M.; Vinardell, M.P.; Solans, C.; Vauthier, C. Interactions of PLGA Nanoparticles with Blood Components: Protein Adsorption, Coagulation, Activation of the Complement System and Hemolysis Studies. Nanoscale 2015, 7, 6045–6058. [Google Scholar] [CrossRef]
- Vuong, Q.L.; Gillis, P.; Roch, A.; Gossuin, Y. Magnetic Resonance Relaxation Induced by Superparamagnetic Particles Used as Contrast Agents in Magnetic Resonance Imaging: A Theoretical Review. WIREs Nanomed. Nanobiotechnology 2017, 9, e1468. [Google Scholar] [CrossRef]
- Wang, Y.-X.J. Superparamagnetic Iron Oxide Based MRI Contrast Agents: Current Status of Clinical Application. Quant Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Karageorgis, A.; Dufort, S.; Sancey, L.; Henry, M.; Hirsjärvi, S.; Passirani, C.; Benoit, J.-P.; Gravier, J.; Texier, I.; Montigon, O.; et al. An MRI-Based Classification Scheme to Predict Passive Access of 5 to 50-nm Large Nanoparticles to Tumors. Sci. Rep. 2016, 6, 21417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazile, D.V.; Ropert, C.; Huve, P.; Verrecchia, T.; Marlard, M.; Frydman, A.; Veillard, M.; Spenlehauer, G. Body Distribution of Fully Biodegradable [14C]-Poly(Lactic Acid) Nanoparticles Coated with Albumin after Parenteral Administration to Rats. Biomaterials 1992, 13, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, V.A.; Vodopyanov, S.S.; Vlasova, K.Y.; Potashnikova, D.M.; Melnikov, P.A.; Vishnevskiy, D.A.; Garanina, A.S.; Valikhov, M.P.; Lipatova, A.V.; Chekhonin, V.P.; et al. Intravital Imaging of Liposome Behavior upon Repeated Administration: A Step towards the Development of Liposomal Companion Diagnostic for Cancer Nanotherapy. J. Control. Release 2021, 330, 244–256. [Google Scholar] [CrossRef]
- Fang, S.; Lin, J.; Li, C.; Huang, P.; Hou, W.; Zhang, C.; Liu, J.; Huang, S.; Luo, Y.; Fan, W.; et al. Dual-Stimuli Responsive Nanotheranostics for Multimodal Imaging Guided Trimodal Synergistic Therapy. Small 2017, 13, 1602580. [Google Scholar] [CrossRef] [PubMed]
- Efremova, M.V.; Naumenko, V.A.; Spasova, M.; Garanina, A.S.; Abakumov, M.A.; Blokhina, A.D.; Melnikov, P.A.; Prelovskaya, A.O.; Heidelmann, M.; Li, Z.-A.; et al. Magnetite-Gold Nanohybrids as Ideal All-in-One Platforms for Theranostics. Sci. Rep. 2018, 8, 11295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Chen, S.; Duo, Y.; Zhu, Y.; Fan, T.; Zou, Q.; Qu, M.; Lin, Z.; Zhao, J.; Li, Y.; et al. Biocompatible Two-Dimensional Titanium Nanosheets for Multimodal Imaging-Guided Cancer Theranostics. ACS Appl Mater. Interfaces 2019, 11, 22129–22140. [Google Scholar] [CrossRef]
- Đorđević, S.; Medel Gonzalez, M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current Hurdles to the Translation of Nanomedicines from Bench to the Clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef]
- Harney, A.S.; Arwert, E.N.; Entenberg, D.; Wang, Y.; Guo, P.; Qian, B.-Z.; Oktay, M.H.; Pollard, J.W.; Jones, J.G.; Condeelis, J.S. Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage-Derived VEGFA. Cancer Discov. 2015, 5, 932–943. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.-M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic Understanding of in Vivo Protein Corona Formation on Polymeric Nanoparticles and Impact on Pharmacokinetics. Nat. Commun. 2017, 8, 777. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.A.; Weissleder, R. Imaging the Pharmacology of Nanomaterials by Intravital Microscopy: Toward Understanding Their Biological Behavior. Adv. Drug Deliv. Rev. 2017, 113, 61–86. [Google Scholar] [CrossRef]
- Jiang, W.; Huang, Y.; An, Y.; Kim, B.Y.S. Remodeling Tumor Vasculature to Enhance Delivery of Intermediate-Sized Nanoparticles. ACS Nano 2015, 9, 8689–8696. [Google Scholar] [CrossRef]
- Haviv, A.H.; Grenèche, J.-M.; Lellouche, J.-P. Aggregation Control of Hydrophilic Maghemite (γ-Fe2O3) Nanoparticles by Surface Doping Using Cerium Atoms. J. Am. Chem. Soc. 2010, 132, 12519–12521. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, A.M. Standards for Photoluminescence Quantum Yield Measurements in Solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.T.R.; Winfield, S.A.; Miller, J.N. Relative Fluorescence Quantum Yields Using a Computer-Controlled Luminescence Spectrometer. Analyst 1983, 108, 1067. [Google Scholar] [CrossRef]
- Kim, J.-B.; Urban, K.; Cochran, E.; Lee, S.; Ang, A. Non-Invasive Detection of a Small Number of Bioluminescent Cancer Cells In Vivo. PLoS ONE 2010, 5, 9364. [Google Scholar] [CrossRef] [PubMed]
- Vercauteren, D.; Vandenbroucke, R.E.; Jones, A.T.; Rejman, J.; Demeester, J.; de Smedt, S.C.; Sanders, N.N.; Braeckmans, K. The Use of Inhibitors to Study Endocytic Pathways of Gene Carriers: Optimization and Pitfalls. Mol. Ther. 2010, 18, 561–569. [Google Scholar] [CrossRef] [PubMed]












| Nanoparticle Type | Hydrodynamic Diameter * (nm) | PDI | Zeta Potential (mV) | Drug Loading (%) | Drug Encapsulation Efficiency (%) |
|---|---|---|---|---|---|
| PLGA-Cy5 | 96 ± 1 | 0.146 ± 0.027 | −30.1 ± 0.3 | - | - |
| PLGA-Cy3 | 96 ± 1 | 0.117 ± 0.011 | −33.4 ± 1.5 | - | - |
| Dox-PLGA-Cy5 | 88 ± 1 | 0.073 ± 0.014 | −14.4 ± 0.1 | 7.6 ± 0.1 | 96.6 |
| hMNP-FITC | 133 ± 1 | 0.108 ± 0.008 | −36.3 ± 0.5 | - | - |
| hMNP-Cy3 | 144 ± 1 | 0.108 ± 0.014 | −37.6 ± 0.5 | - | - |
| hMNP | 109.3 ± 2.5 | 0.133 ± 0.029 | −38.2 ± 2.2 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinovskaya, J.; Salami, R.; Valikhov, M.; Vadekhina, V.; Semyonkin, A.; Semkina, A.; Abakumov, M.; Harel, Y.; Levy, E.; Levin, T.; et al. Supermagnetic Human Serum Albumin (HSA) Nanoparticles and PLGA-Based Doxorubicin Nanoformulation: A Duet for Selective Nanotherapy. Int. J. Mol. Sci. 2023, 24, 627. https://doi.org/10.3390/ijms24010627
Malinovskaya J, Salami R, Valikhov M, Vadekhina V, Semyonkin A, Semkina A, Abakumov M, Harel Y, Levy E, Levin T, et al. Supermagnetic Human Serum Albumin (HSA) Nanoparticles and PLGA-Based Doxorubicin Nanoformulation: A Duet for Selective Nanotherapy. International Journal of Molecular Sciences. 2023; 24(1):627. https://doi.org/10.3390/ijms24010627
Chicago/Turabian StyleMalinovskaya, Julia, Rawan Salami, Marat Valikhov, Veronika Vadekhina, Aleksey Semyonkin, Alevtina Semkina, Maxim Abakumov, Yifat Harel, Esthy Levy, Tzuriel Levin, and et al. 2023. "Supermagnetic Human Serum Albumin (HSA) Nanoparticles and PLGA-Based Doxorubicin Nanoformulation: A Duet for Selective Nanotherapy" International Journal of Molecular Sciences 24, no. 1: 627. https://doi.org/10.3390/ijms24010627