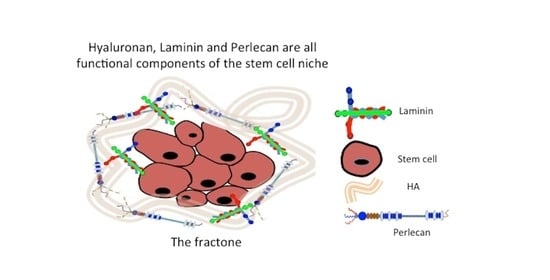
Fractone Stem Cell Niche Components Provide Intuitive Clues in the Design of New Therapeutic Procedures/Biomatrices for Neural Repair
Abstract
:1. Introduction
2. Neuroprogenitor Stem Cell Niches and the Cell Regulatory Environment Provided by ECM Components
3. Development of HA Hydrogel Cell Delivery and Therapeutic Biomatrices for Tissue Repair
Application of HA as a Delivery Vehicle for Olfactory Ensheathing Cells ± Mesenchymal Stem Cells from a Number of Tissues for Neural Repair
4. Application of HS Containing Biomatrices for Neural Repair
4.1. Harnessing Cell Instructive Properties of Perlecan’s HS Side Chains in Repair Biology
4.2. Development of Artificial Neural Stem Cell Niches
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delpech, B.; Delpech, A.; Brückner, G.; Girard, N.; Maingonnat, C. Hyaluronan and hyaluronectin in the nervous system. Ciba Found. Symp. 1989, 143, 208–285. [Google Scholar]
- Oohashi, T.; Edamatsu, M.; Bekku, Y.; Carulli, D. The hyaluronan and proteoglycan link proteins: Organizers of the brain extracellular matrix and key molecules for neuronal function and plasticity. Exp. Neurol. 2015, 274 Pt B, 134–144. [Google Scholar] [CrossRef]
- Suttkus, A.; Morawski, M.; Arendt, T. Protective Properties of Neural Extracellular Matrix. Mol. Neurobiol. 2016, 53, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Bosiacki, M.; Gąssowska-Dobrowolska, M.; Kojder, K.; Fabiańska, M.; Jeżewski, D.; Gutowska, I.; Lubkowska, A. Perineuronal Nets and Their Role in Synaptic Homeostasis. Int. J. Mol. Sci. 2019, 20, 4108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, A.; Sherman, L.S. Diverse Roles for Hyaluronan and Hyaluronan Receptors in the Developing and Adult Nervous System. Int. J. Mol. Sci. 2020, 21, 5988. [Google Scholar] [CrossRef] [PubMed]
- Arranz, A.; Perkins, K.L.; Irie, F.; Lewis, D.P.; Hrabe, J.; Xiao, F.; Itano, N.; Kimata, K.; Hrabetova, S.; Yamaguchi, Y. Hyaluronan deficiency due to Has3 knock-out causes altered neuronal activity and seizures via reduction in brain extracellular space. J. Neurosci. 2014, 34, 6164–6176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins, K.; Arranz, A.M.; Yamaguchi, Y.; Hrabetova, S. Brain extracellular space, hyaluronan, and the prevention of epileptic seizures. Rev. Neurosci. 2017, 28, 869–892. [Google Scholar] [CrossRef]
- Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978, 4, 7–25. [Google Scholar]
- Smart, I. The subependymal layer of the mouse brain and its cell production as shown by autography after [H3]-thymidine injection. J. Comp. Neurol. 1961, 116, 325–347. [Google Scholar] [CrossRef]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef]
- Preston, M.; Sherman, L.S. Neural Stem Cell Niches: Critical Roles for the Hyaluronan-Based Extracellular Matrix in Neural Stem Cell Proliferation and Differentiation. Front. Biosci. 2011, 3, 1165–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decimo, I.; Bifari, F.; Krampera, M.; Fumagalli, G. Neural stem cell niches in health and diseases. Curr. Pharm. Des. 2012, 18, 1755–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, R.; Gritti, A.; Bonfanti, L.; Vescovi, A.L. Neural stem cells: An overview. Circ. Res. 2003, 92, 598–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parati, E.; Pozzi, S.; Ottolina, A.; Onofrj, M.; Bez, A.; Pagano, S.F. Neural stem cells: An overview. J. Endocrinol. Investig. 2004, 27 (Suppl. S6), 64–67. [Google Scholar]
- Ekonomou, A.; Cedar, S.H.; Ballard, C.G.; Minger, S.L. Stem-cells: Prospects for treating dementia. Br. J. Neurosci. Nurs. 2008, 4, 220–222. [Google Scholar] [CrossRef]
- Pennings, S.; Liu, K.J.; Qian, H. The Stem Cell Niche: Interactions between Stem Cells and Their Environment. Stem Cells Int. 2018, 2018, 4879379. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Kiyozumi, D.; Futaki, S.; Nakano, I.; Shimono, C.; Kaneko, N.; Ikawa, M.; Okabe, M.; Sawamoto, K.; Sekiguchi, K. Ventricular-subventricular zone fractones are speckled basement membranes that function as a neural stem cell niche. Mol. Biol. Cell 2019, 30, 56–68. [Google Scholar] [CrossRef]
- Morante-Redolat, J.; Porlan, E. Neural Stem Cell Regulation by Adhesion Molecules Within the Subependymal Niche. Front. Cell Dev. Biol. 2019, 7, 102. [Google Scholar] [CrossRef]
- Rai, S.; Wang, L. Regulatory functions of heparin sulphate in stem cell self renewal and differentiation. In Proteoglycans in Stem Cells; Biology of Extracellular matrix; Gotte, M., Forsberg-Nilsson, K., Eds.; Springer Nature: Cham, Switzerland, 2021; Volume 9, pp. 95–110. [Google Scholar]
- Fuentealba, L.; Obernier, K.; Alvarez-Buylla, A. Adult neural stem cells bridge their niche. Cell Stem Cell 2012, 10, 698–708. [Google Scholar] [CrossRef] [Green Version]
- Mandelbrot, B. The Fractal Geometry of Nature; Freeman: New York, NY, USA, 1983. [Google Scholar]
- Meletis, K.; Barnabé-Heider, F.; Carlén, M.; Evergren, E.; Tomilin, N.; Shupliakov, O.; Frisén, J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008, 6, e182. [Google Scholar] [CrossRef]
- George, N.; Geller, H.M. Extracellular matrix and traumatic brain injury. J. Neurosci. Res. 2018, 96, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, S.; Hamilton, L.K.; Vaugeois, A.; Beaudoin, S.; Breault-Dugas, C.; Pineau, I.; Lévesque, S.A.; Grégoire, C.A.; Fernandes, K.J. Central canal ependymal cells proliferate extensively in response to traumatic spinal cord injury but not demyelinating lesions. PLoS ONE 2014, 9, e85916. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Kitagawa, H. Formation and remodeling of the brain extracellular matrix in neural plasticity: Roles of chondroitin sulfate and hyaluronan. Biochim. Biophys. Acta Gen. Sub. J. 2017, 1861, 2420–2434. [Google Scholar] [CrossRef] [PubMed]
- Shabani, Z.; Ghadiri, T.; Karimipour, M.; Sadigh-Eteghad, S.; Mahmoudi, J.; Mehrad, H.; Farhoudi, M. Modulatory properties of extracellular matrix glycosaminoglycans and proteoglycans on neural stem cells behavior: Highlights on regenerative potential and bioactivity. Int. J. Biol. Macromol. 2021, 171, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Sherman, L.; Matsumoto, S.; Su, W.; Srivastava, T.; Back, S.A. Hyaluronan Synthesis, Catabolism, and Signaling in Neurodegenerative Diseases. Int. J. Cell Biol. 2015, 2015, 368584. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Hu, J.; Ralls, S.; Kitamura, T.; Loh, Y.P.; Yang, Y.; Mukouyama, Y.S.; Ahn, S.T. The molecular profiles of neural stem cell niche in the adult subventricular zone. PLoS ONE 2012, 7, e50501. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.; Alvarez-Buylla, A. Adult neural stem cells stake their ground. Trends Neurosci. 2014, 37, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Redmond, S.; Figueres-Oñate, M.; Obernier, K.; Nascimento, M.A.; Parraguez, J.I.; López-Mascaraque, L.; Fuentealba, L.C.; Alvarez-Buylla, A. Development of Ependymal and Postnatal Neural Stem Cells and Their Origin from a Common Embryonic Progenitor. Cell Rep. 2019, 27, 429–441.e3. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Yadav, C.B.; Tabassum, N.; Bajpeyee, A.K.; Verma, V. Stem cell niche: Dynamic neighbor of stem cells. Eur. J. Cell Biol. 2019, 98, 65–73. [Google Scholar] [CrossRef]
- Mercier, F. Fractones: Extracellular matrix niche controlling stem cell fate and growth factor activity in the brain in health and disease. Cell. Mol. Life Sci. 2016, 73, 4661–4674. [Google Scholar] [CrossRef]
- Nascimento, M.; Sorokin, L.; Coelho-Sampaio, T. Fractone Bulbs Derive from Ependymal Cells and Their Laminin Composition Influence the Stem Cell Niche in the Subventricular Zone. J. Neurosci. 2018, 38, 3880–3889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regalado-Santiago, C.; Juárez-Aguilar, E.; Olivares-Hernández, J.D.; Tamariz, E. Mimicking Neural Stem Cell Niche by Biocompatible Substrates. Stem Cells Int. 2016, 2016, 1513285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioannidis, K.; Angelopoulos, I.; Gakis, G.; Karantzelis, N.; Spyroulias, G.A.; Lygerou, Z.; Taraviras, S. 3D Reconstitution of the Neural Stem Cell Niche: Connecting the Dots. Front. Bioeng. Biotechnol. 2021, 9, 705470. [Google Scholar] [CrossRef]
- Relucio, J.; Menezes, M.J.; Miyagoe-Suzuki, Y.; Takeda, S.; Colognato, H. Laminin regulates postnatal oligodendrocyte production by promoting oligodendrocyte progenitor survival in the subventricular zone. Glia 2012, 60, 1451–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerever, A.; Mercier, F.; Nonaka, R.; de Vega, S.; Oda, Y.; Zalc, B.; Okada, Y.; Hattori, N.; Yamada, Y.; Arikawa-Hirasawa, E. Perlecan is required for FGF-2 signaling in the neural stem cell niche. Stem Cell Res. 2014, 12, 492–505. [Google Scholar] [CrossRef]
- Smith, M.; Melrose, J. Perlecan Delineates stem cell niches in Human Foetal Hip, Knee and Elbow Cartilage Rudiments and has potential roles in the regulation of Stem cell Differentiation. J. Stem Cell Res. Dev. Ther. 2016, 3, 9–16. [Google Scholar]
- Morrison, S.; Spradling, A.C. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef] [Green Version]
- Kerever, A.; Schnack, J.; Vellinga, D.; Ichikawa, N.; Moon, C.; Arikawa-Hirasawa, E.; Efird, J.T.; Mercier, F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells 2007, 25, 2146–2157. [Google Scholar] [CrossRef]
- Kerever, A.; Yamada, T.; Suzuki, Y.; Mercier, F.; Arikawa-Hirasawa, E. Fractone aging in the subventricular zone of the lateral ventricle. J. Chem. Neuroanat. 2015, 66–67, 52–60. [Google Scholar] [CrossRef]
- Yamada, T.; Kerever, A.; Yoshimura, Y.; Suzuki, Y.; Nonaka, R.; Higashi, K.; Toida, T.; Mercier, F.; Arikawa-Hirasawa, E. Heparan sulfate alterations in extracellular matrix structures and fibroblast growth factor-2 signaling impairment in the aged neurogenic niche. J. Neurochem. 2017, 142, 534–544. [Google Scholar] [CrossRef]
- Mercier, F.; Cho Kwon, Y.; Kodama, R. Meningeal/vascular alterations and loss of extracellular matrix in the neurogenic zone of adult BTBR T+ tf/J mice, animal model for autism. Neurosci. Lett. 2011, 498, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Mercier, F.; Kwon, Y.C.; Douet, V. Hippocampus/amygdala alterations, loss of heparan sulfates, fractones and ventricle wall reduction in adult BTBR T+ tf/J mice, animal model for autism. Neurosci. Lett. 2012, 506, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Matta, A.; Erwin, M.; Roberts, S.; Gruber, H.E.; Hanley, E.N., Jr.; Little, C.B.; Melrose, J. Cell Clusters Are Indicative of Stem Cell Activity in the Degenerate Intervertebral Disc: Can Their Properties Be Manipulated to Improve Intrinsic Repair of the Disc? Stem Cells Dev. 2018, 27, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.; Hughes, C.E.; Smith, S.M.; Caterson, B.; Little, C.B.; Melrose, J. The CS Sulfation Motifs 4C3, 7D4, 3B3[-]; and Perlecan Identify Stem Cell Populations and Their Niches, Activated Progenitor Cells and Transitional Areas of Tissue Development in the Fetal Human Elbow. Stem Cells Dev. 2016, 25, 836–847. [Google Scholar] [CrossRef]
- Melrose, J.; Hayes, A.J.; Bix, G. The CNS/PNS Extracellular Matrix Provides Instructive Guidance Cues to Neural Cells and Neuroregulatory Proteins in Neural Development and Repair. Int. J. Mol. Sci. 2021, 22, 5583. [Google Scholar] [CrossRef]
- Collins, M.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Sodhi, H.; Panitch, A. Glycosaminoglycans in Tissue Engineering: A Review. Biomolecules 2020, 11, 29. [Google Scholar] [CrossRef]
- Voigt, J.; Driver, V.R. Hyaluronic acid derivatives and their healing effect on burns, epithelial surgical wounds, and chronic wounds: A systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen. 2012, 20, 317–331. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 2041731417726464. [Google Scholar] [CrossRef] [Green Version]
- Kotla, N.; Bonam, S.R.; Rasala, S.; Wankar, J.; Bohara, R.A.; Bayry, J.; Rochev, Y.; Pandit, A. Recent advances and prospects of hyaluronan as a multifunctional therapeutic system. J. Control. Release 2021, 336, 598–620. [Google Scholar] [CrossRef]
- Li, F.; Ducker, M.; Sun, B.; Szele, F.G.; Czernuszka, J.T. Interpenetrating polymer networks of collagen, hyaluronic acid, and chondroitin sulfate as scaffolds for brain tissue engineering. Acta Biomater. 2020, 112, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Spector, M. Development of hyaluronic acid-based scaffolds for brain tissue engineering. Acta Biomater. 2009, 5, 2371–2384. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen- and hyaluronic acid-based hydrogels and their biomedical applications. Mater. Sci. Eng. R Rep. 2021, 146, 100641. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.; Melrose, J. Aggrecan, the Primary Weight-Bearing Cartilage Proteoglycan, Has Context-Dependent, Cell-Directive Properties in Embryonic Development and Neurogenesis: Aggrecan Glycan Side Chain Modifications Convey Interactive Biodiversity. Biomolecules 2020, 10, 1244. [Google Scholar] [CrossRef]
- Lyu, Y.; Xie, J.; Liu, Y.; Xiao, M.; Li, Y.; Yang, J.; Yang, J.; Liu, W. Injectable Hyaluronic Acid Hydrogel Loaded with Functionalized Human Mesenchymal Stem Cell Aggregates for Repairing Infarcted Myocardium. ACS Biomater. Sci. Eng. 2020, 6, 6926–6937. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, L.; Kauppila, M.; Samanta, S.; Parihar, V.S.; Ilmarinen, T.; Miettinen, S.; Oommen, O.P.; Skottman, H. Tissue adhesive hyaluronic acid hydrogels for sutureless stem cell delivery and regeneration of corneal epithelium and stroma. Biomaterials 2019, 225, 119516. [Google Scholar] [CrossRef]
- Gaston, J.; Thibeault, S.L. Hyaluronic acid hydrogels for vocal fold wound healing. Biomatter 2013, 3, e23799. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Hu, S.; Yang, H.; Li, Z.; Huang, K.; Su, T.; Wang, S.; Cheng, K. Hyaluronic Acid Hydrogel Integrated with Mesenchymal Stem Cell-Secretome to Treat Endometrial Injury in a Rat Model of Asherman’s Syndrome. Adv. Healthc. Mater. 2019, 8, e1900411. [Google Scholar] [CrossRef]
- Silva, C.; Babo, P.S.; Gulino, M.; Costa, L.; Oliveira, J.M.; Silva-Correia, J.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Injectable and tunable hyaluronic acid hydrogels releasing chemotactic and angiogenic growth factors for endodontic regeneration. Acta Biomater. 2018, 77, 155–171. [Google Scholar] [CrossRef] [Green Version]
- Soto, J.; Ding, X.; Wang, A.; Song, L. Neural crest-like stem cells for tissue regeneration. Stem Cells Transl. Med. 2021, 10, 681–693. [Google Scholar] [CrossRef]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.; Strymecka, P.; Stanaszek, L.; Silva-Correia, J.; Drela, K.; Fiedorowicz, M.; Malysz-Cymborska, I.; Rogujski, P.; Janowski, M.; Reis, R.L.; et al. Methacrylated gellan gum and hyaluronic acid hydrogel blends for image-guided neurointerventions. J. Mater. Chem. B 2020, 8, 5928–5937. [Google Scholar] [CrossRef] [PubMed]
- Borzacchiello, A.; Russo, L.; Malle, B.M.; Schwach-Abdellaoui, K.; Ambrosio, L. Hyaluronic Acid Based Hydrogels for Regenerative Medicine Applications. Biomed Res. Int. 2015, 2015, 871218. [Google Scholar] [CrossRef]
- Wang, X.; He, J.; Wang, Y.; Cui, F.-Z. Hyaluronic acid-based scaffold for central neural tissue engineering. Interface Focus 2012, 2, 278–291. [Google Scholar] [CrossRef] [Green Version]
- Ehsanipour, A.; Nguyen, T.; Aboufadel, T.; Sathialingam, M.; Cox, P.; Xiao, W.; Walthers, C.M.; Seidlits, S.K. Injectable, Hyaluronic Acid-Based Scaffolds with Macroporous Architecture for Gene Delivery. Cell. Mol. Bioeng. 2019, 12, 399–413. [Google Scholar] [CrossRef]
- Zor, F.; Deveci, M.; Kilic, A.; Ozdag, M.F.; Kurt, B.; Sengezer, M.; Sönmez, T.T. Effect of VEGF gene therapy and hyaluronic acid film sheath on peripheral nerve regeneration. Microsurgery 2014, 34, 209–216. [Google Scholar] [CrossRef]
- Farrukh, A.; Ortega, F.; Fan, W.; Marichal, N.; Paez, J.I.; Berninger, B.; Campo, A.D.; Salierno, M.J. Bifunctional Hydrogels Containing the Laminin Motif IKVAV Promote Neurogenesis. Stem Cell Rep. 2017, 9, 1432–1440. [Google Scholar] [CrossRef] [Green Version]
- Freitas, V.M.; Vilas-Boas, V.F.; Pimenta, D.C.; Loureiro, V.; Juliano, M.A.; Carvalho, M.R.; Pinheiro, J.J.; Camargo, A.C.; Moriscot, A.S.; Hoffman, M.P.; et al. SIKVAV, a laminin alpha1-derived peptide, interacts with integrins and increases protease activity of a human salivary gland adenoid cystic carcinoma cell line through the ERK 1/2 signaling pathway. Am. J. Pathol. 2007, 171, 124–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Tian, W.M.; Yu, X.; Cui, F.Z.; Hou, S.P.; Xu, Q.Y.; Lee, I.S. Hyaluronic acid hydrogels with IKVAV peptides for tissue repair and axonal regeneration in an injured rat brain. Biomed. Mater. 2007, 2, S142–S146. [Google Scholar] [CrossRef]
- Zaviskova, K.; Tukmachev, D.; Dubisova, J.; Vackova, I.; Hejcl, A.; Bystronova, J.; Pravda, M.; Scigalkova, I.; Sulakova, R.; Velebny, V.; et al. Injectable hydroxyphenyl derivative of hyaluronic acid hydrogel modified with RGD as scaffold for spinal cord injury repair. J. Biomed. Mater. Res. A 2018, 106, 1129–1140. [Google Scholar] [CrossRef]
- Farrell, K.; Joshi, J.; Kothapalli, C.R. Injectable uncrosslinked biomimetic hydrogels as candidate scaffolds for neural stem cell delivery. J. Biomed. Mater. Res. A 2017, 105, 790–805. [Google Scholar] [CrossRef]
- Hou, S.; Xu, Q.; Tian, W.; Cui, F.; Cai, Q.; Ma, J.; Lee, I.S. The repair of brain lesion by implantation of hyaluronic acid hydrogels modified with laminin. J. Neurosci. Methods 2005, 148, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, H.; Zou, Y.; Xue, W.; Zhuang, Y.; Gu, R.; Shen, H.; Dai, J. Optimized, visible light-induced crosslinkable hybrid gelatin/hyaluronic acid scaffold promotes complete spinal cord injury repair. Biomed. Mater. 2022, 17, 024104. [Google Scholar] [CrossRef]
- Lin, C.; Ekblad-Nordberg, Å.; Michaëlsson, J.; Götherström, C.; Hsu, C.C.; Ye, H.; Johansson, J.; Rising, A.; Sundström, E.; Åkesson, E. In Vitro Study of Human Immune Responses to Hyaluronic Acid Hydrogels, Recombinant Spidroins and Human Neural Progenitor Cells of Relevance to Spinal Cord Injury Repair. Cells 2021, 10, 1713. [Google Scholar] [CrossRef] [PubMed]
- Entekhabi, E.; Haghbin Nazarpak, M.; Shafieian, M.; Mohammadi, H.; Firouzi, M.; Hassannejad, Z. Fabrication and in vitro evaluation of 3D composite scaffold based on collagen/hyaluronic acid sponge and electrospun polycaprolactone nanofibers for peripheral nerve regeneration. J. Biomed. Mater. Res. A 2021, 109, 300–312. [Google Scholar] [CrossRef]
- Tian, W.; Hou, S.P.; Ma, J.; Zhang, C.L.; Xu, Q.Y.; Lee, I.S.; Li, H.D.; Spector, M.; Cui, F.Z. Hyaluronic acid-poly-D-lysine-based three-dimensional hydrogel for traumatic brain injury. Tissue Eng. 2005, 11, 513–525. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Y.T.; Zu, Z.H.; Ju, R.K.; Guo, M.Y.; Wang, X.M.; Xu, Q.Y.; Cui, F.Z. Combination of hyaluronic acid hydrogel scaffold and PLGA microspheres for supporting survival of neural stem cells. Pharm. Res. 2011, 28, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Roche, P.; Alekseeva, T.; Widaa, A.; Ryan, A.; Matsiko, A.; Walsh, M.; Duffy, G.P.; O’Brien, F.J. Olfactory Derived Stem Cells Delivered in a Biphasic Conduit Promote Peripheral Nerve Repair In Vivo. Stem Cells Transl. Med. 2017, 6, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Hertegård, S.; Nagubothu, S.R.; Malmström, E.; Ström, C.E.; Tolf, A.; Davies, L.C.; Le Blanc, K. Hyaluronan Hydrogels for the Local Delivery of Mesenchymal Stromal Cells to the Injured Vocal Fold. Stem Cells Dev. 2019, 28, 1177–1190. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, G.; Agiwal, S.; Srivastava, A. Hyaluronic acid containing scaffolds ameliorate stem cell function for tissue repair and regeneration. Int. J. Biol. Macromol. 2020, 165 Pt A, 388–401. [Google Scholar] [CrossRef]
- Shendi, D.; Dede, A.; Yin, Y.; Wang, C.; Valmikinathan, C.; Jain, A. Tunable, bioactive protein conjugated hyaluronic acid hydrogel for neural engineering applications. J. Mater. Chem B 2016, 4, 2803–2818. [Google Scholar] [CrossRef]
- Entekhabi, E.; Haghbin Nazarpak, M.; Moztarzadeh, F.; Sadeghi, A. Design and manufacture of neural tissue engineering scaffolds using hyaluronic acid and polycaprolactone nanofibers with controlled porosity. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 380–387. [Google Scholar] [CrossRef]
- Radtke, C.; Kocsis, J.D. Olfactory-ensheathing cell transplantation for peripheral nerve repair: Update on recent developments. Cells Tissues Organs 2014, 200, 48–58. [Google Scholar] [CrossRef]
- Reshamwala, R.; Shah, M.; St John, J.; Ekberg, J. Survival and Integration of Transplanted Olfactory Ensheathing Cells are Crucial for Spinal Cord Injury Repair: Insights from the Last 10 Years of Animal Model Studies. Cell Transplant. 2019, 28 (Suppl. S1), 132S–159S. [Google Scholar] [CrossRef]
- Guérout, N.; Paviot, A.; Bon-Mardion, N.; Duclos, C.; Genty, D.; Jean, L.; Boyer, O.; Marie, J.P. Co-transplantation of olfactory ensheathing cells from mucosa and bulb origin enhances functional recovery after peripheral nerve lesion. PLoS ONE 2011, 6, e22816. [Google Scholar] [CrossRef]
- Radtke, C.; Wewetzer, K.; Reimers, K.; Vogt, P.M. Transplantation of olfactory ensheathing cells as adjunct cell therapy for peripheral nerve injury. Cell Transplant. 2011, 20, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Gilmour, A.; Reshamwala, R.; Wright, A.A.; Ekberg, J.A.K.; St John, J.A. Optimizing Olfactory Ensheathing Cell Transplantation for Spinal Cord Injury Repair. J. Neurotrauma 2020, 37, 817–829. [Google Scholar] [CrossRef]
- Liu, Z.; Martin, L.J. Pluripotent fates and tissue regenerative potential of adult olfactory bulb neural stem and progenitor cells. J. Neurotrauma 2004, 21, 1479–1499. [Google Scholar] [CrossRef] [Green Version]
- Mackay-Sim, A.; St John, J.A. Olfactory ensheathing cells from the nose: Clinical application in human spinal cord injuries. Exp. Neurol. 2011, 229, 174–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, R.; Murtaza, M.; Velasquez, J.T.; Todorovic, M.; Rayfield, A.; Ekberg, J.; Barton, M.; St John, J. Olfactory Ensheathing Cells for Spinal Cord Injury: Sniffing Out the Issues. Cell Transplant. 2018, 27, 879–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdani, S.; Pedram, M.; Hafizi, M.; Kabiri, M.; Soleimani, M.; Dehghan, M.M.; Jahanzad, I.; Gheisari, Y.; Hashemi, S.M. A comparison between neurally induced bone marrow derived mesenchymal stem cells and olfactory ensheathing glial cells to repair spinal cord injuries in rat. Tissue Cell 2012, 44, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ao, Q.; Gong, K.; Zuo, H.; Gong, Y.; Zhang, X. Synergistic effect of neural stem cells and olfactory ensheathing cells on repair of adult rat spinal cord injury. Cell Transplant. 2010, 19, 1325–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Liu, S.J.; Dan, Q.Q.; Wang, Y.P.; Lin, N.; Lv, L.Y.; Zou, Y.; Liu, S.; Zhou, X.; Wang, T.H. Combined Bone Mesenchymal Stem Cell and Olfactory Ensheathing Cell Transplantation Promotes Neural Repair Associated with CNTF Expression in Traumatic Brain-Injured Rats. Cell Transplant. 2015, 24, 1533–1544. [Google Scholar] [CrossRef] [Green Version]
- Augestad, I.; Nyman, A.K.G.; Costa, A.I.; Barnett, S.C.; Sandvig, A.; Håberg, A.K.; Sandvig, I. Effects of Neural Stem Cell and Olfactory Ensheathing Cell Co-transplants on Tissue Remodelling After Transient Focal Cerebral Ischemia in the Adult Rat. Neurochem. Res. 2017, 42, 1599–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, W.T.; Gong, C.R.; Li, C.; Shi, M. Combination of olfactory ensheathing cells and human umbilical cord mesenchymal stem cell-derived exosomes promotes sciatic nerve regeneration. Neural Regen. Res. 2020, 15, 1803–1911. [Google Scholar]
- Ferrero-Gutierrez, A.; Menendez-Menendez, Y.; Alvarez-Viejo, M.; Meana, A.; Otero, J. New serum-derived albumin scaffold seeded with adipose-derived stem cells and olfactory ensheathing cells used to treat spinal cord injured rats. Histol. Histopathol. 2013, 28, 89–100. [Google Scholar] [PubMed]
- Kabiri, M.; Oraee-Yazdani, S.; Shafiee, A.; Hanaee-Ahvaz, H.; Dodel, M.; Vaseei, M.; Soleimani, M. Neuroregenerative effects of olfactory ensheathing cells transplanted in a multi-layered conductive nanofibrous conduit in peripheral nerve repair in rats. J. Biomed. Sci. 2015, 22, 35. [Google Scholar] [CrossRef]
- Mutepfa, A.; Hardy, J.G.; Adams, C.F. Electroactive Scaffolds to Improve Neural Stem Cell Therapy for Spinal Cord Injury. Front. Med. Technol. 2022, 4, 693438. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.; Whitelock, J.; Melrose, J. Regulation of FGF-2, FGF-18 and Transcription Factor Activity by Perlecan in the Maturational Development of Transitional Rudiment and Growth Plate Cartilages and in the Maintenance of Permanent Cartilage Homeostasis. Int. J. Mol. Sci. 2022, 23, 1934. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.; Farrugia, B.L.; Biose, I.J.; Bix, G.J.; Melrose, J. Perlecan, A Multi-Functional, Cell-Instructive, Matrix-Stabilizing Proteoglycan with Roles in Tissue Development Has Relevance to Connective Tissue Repair and Regeneration. Front. Cell Dev. Biol. 2022, 10, 856261. [Google Scholar] [CrossRef]
- Girós, A.; Morante, J.; Gil-Sanz, C.; Fairén, A.; Costell, M. Perlecan controls neurogenesis in the developing telencephalon. BMC Dev. Biol. 2007, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Arikawa-Hirasawa, E. Impact of the Heparan Sulfate Proteoglycan Perlecan on Human Disease and Health. Am. J. Physiol. Cell Physiol. 2022. [Google Scholar] [CrossRef]
- Ford-Perriss, M.; Turner, K.; Guimond, S.; Apedaile, A.; Haubeck, H.D.; Turnbull, J.; Murphy, M. Localisation of specific heparan sulfate proteoglycans during the proliferative phase of brain development. Dev. Dyn. 2003, 227, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J. Perlecan, a modular instructive proteoglycan with diverse functional properties. Int. J. Biochem. Cell Biol. 2020, 128, 105849. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, F.; Sadeghi, M.; Rajaei, F. Induction of perlecan expression and neural cell proliferation by FGF-2 in the developing cerebral cortex: An in vivo study. J. Mol. Neurosci. 2011, 45, 87–93. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Cerrato, V.; Fucà, E.; Parmigiani, E.; Buffo, A.; Leto, K. Sonic hedgehog patterning during cerebellar development. Cell. Mol. Life Sci. 2016, 73, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Ikeya, M.; Lee, S.M.; Johnson, J.E.; McMahon, A.P.; Takada, S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature 1997, 389, 966–970. [Google Scholar] [CrossRef]
- Casper, C.; Yang, W.; Farach-Carson, M.C.; Rabolt, J.F. Coating electrospun collagen and gelatin fibers with perlecan domain I for increased growth factor binding. Biomacromolecules 2007, 8, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Yang, W.; Kirn-Safran, C.B.; Farach-Carson, M.C.; Jia, X. Perlecan domain I-conjugated, hyaluronic acid-based hydrogel particles for enhanced chondrogenic differentiation via BMP-2 release. Biomaterials 2009, 30, 6964–6975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, P.; McCoy, S.Y.; Jha, A.K.; Yang, W.; Jia, X.; Farach-Carson, M.C.; Kirn-Safran, C.B. Injectable perlecan domain 1-hyaluronan microgels potentiate the cartilage repair effect of BMP2 in a murine model of early osteoarthritis. Biomed. Mater. 2012, 7, 024109. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Gomes, R.R.; Alicknavitch, M., Jr.; Farach-Carson, M.C.; Carson, D.D. Perlecan domain I promotes fibroblast growth factor 2 delivery in collagen I fibril scaffolds. Tissue Eng. 2005, 11, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Bix, G. Perlecan domain V therapy for stroke: A beacon of hope? ACS Chem. Neurosci. 2013, 4, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Marcelo, A.; Bix, G. Investigating the role of perlecan domain V in post-ischemic cerebral angiogenesis. Methods Mol. Biol. 2014, 1135, 331–341. [Google Scholar] [PubMed]
- Trout, A.; Kahle, M.P.; Roberts, J.M.; Marcelo, A.; de Hoog, L.; Boychuk, J.A.; Grupke, S.L.; Berretta, A.; Gowing, E.K.; Boychuk, C.R.; et al. Perlecan Domain-V Enhances Neurogenic Brain Repair After Stroke in Mice. Transl. Stroke Res. 2020, 12, 72–86. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Griffiths, L.R.; Haupt, L.M. Exploiting Heparan Sulfate Proteoglycans in Human Neurogenesis-Controlling Lineage Specification and Fate. Front. Integr. Neurosci. 2017, 11, 28. [Google Scholar] [CrossRef] [Green Version]
- Condomitti, G.; de Wit, J. Heparan Sulfate Proteoglycans as Emerging Players in Synaptic Specificity. Front. Mol. Neurosci. 2018, 11, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Lu, H.; Peixoto, R.T.; Pines, M.K.; Ge, Y.; Oku, S.; Siddiqui, T.J.; Xie, Y.; Wu, W.; Archer-Hartmann, S.; et al. Heparan Sulfate Organizes Neuronal Synapses through Neurexin Partnerships. Cell 2018, 174, 1450–1464.e23. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, R.J.; Chen, M.; Liu, X.Y.; Ma, K.; Xu, H.Y.; Deng, W.S.; Ye, Y.C.; Li, W.X.; Chen, X.Y.; et al. Collagen/heparan sulfate porous scaffolds loaded with neural stem cells improve neurological function in a rat model of traumatic brain injury. Neural Regen. Res. 2021, 16, 1068–1077. [Google Scholar]
- Chen, C.; Zhao, M.L.; Zhang, R.K.; Lu, G.; Zhao, C.Y.; Fu, F.; Sun, H.T.; Zhang, S.; Tu, Y.; Li, X.H. Collagen/heparin sulfate scaffolds fabricated by a 3D bioprinter improved mechanical properties and neurological function after spinal cord injury in rats. J. Biomed Mater. Res. A 2017, 105, 1324–1332. [Google Scholar] [CrossRef]
- Guan, S.; Zhang, X.L.; Lin, X.M.; Liu, T.Q.; Ma, X.H.; Cui, Z.F. Chitosan/gelatin porous scaffolds containing hyaluronic acid and heparan sulfate for neural tissue engineering. J. Biomater. Sci. Polym. Ed. 2013, 24, 999–1014. [Google Scholar] [CrossRef]
- Weißenbruch, K.; Lemma, E.D.; Hippler, M.; Bastmeyer, M. Micro-scaffolds as synthetic cell niches: Recent advances and challenges. Curr. Opin. Biotechnol. 2022, 73, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Barros, D.; Conde-Sousa, E.; Gonçalves, A.M.; Han, W.M.; García, A.J.; Amaral, I.F.; Pêgo, A.P. Engineering hydrogels with affinity-bound laminin as 3D neural stem cell culture systems. Biomater. Sci. 2019, 7, 5338–5349. [Google Scholar] [CrossRef]
- Barros, D.; Amaral, I.F.; Pêgo, A.P. Laminin-Inspired Cell-Instructive Microenvironments for Neural Stem Cells. Biomacromolecules 2020, 21, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Buzanska, L.; Zychowicz, M.; Kinsner-Ovaskainen, A. Bioengineering of the Human Neural Stem Cell Niche: A Regulatory Environment for Cell Fate and Potential Target for Neurotoxicity. Probl. Cell Differ. 2018, 66, 207–230. [Google Scholar]



| HA Hydrogel/Scaffold and Its Properties in Tissue Repair Processes | Ref | |
|---|---|---|
| Injectable HA hydrogel | MSC repair of infarcted myocardium. | [57] |
| Tissue adhesive HA hydrogel | Sutureless stem cell delivery and regeneration of corneal epithelium and stroma. | [58] |
| HA hydrogel | MSC delivery to damaged vocal cord. | [81] |
| HA hydrogel | Treatment of Endometrial Injury in a Rat Model of Asherman’s Syndrome. | [60] |
| Injectable HA hydrogel | Tunable HA hydrogels releasing chemotactic and angiogenic growth factors for endodontic regeneration. | [61] |
| HA scaffold | Scaffolds that improve stem cell functions for tissue repair and regeneration. | [82] |
| Interpenetrating collagen, HA, polymer networks | Scaffolds for brain tissue engineering. | [53] |
| Injectable HA Scaffolds with Macroporous Architecture | Scaffold designed for gene delivery for tissue repair. | [67] |
| Combination of hyaluronic acid hydrogel scaffold and PLGA microspheres | Extended delivery of VEGF and BDNF from PGLA microspheres promotes neural growth. | [79] |
| Divynyl sulfone crosslinked HA | Scaffolds with a range of pore sizes supporting cell migration and neurite extension. | [83] |
| Neurotrophin NGF-HA hydrogel filler cell delivery system | Scaffold filler hydrogel used in combination with olfactory ensheathing cells to repair of a 10 mm gap model of sciatic nerve injury in Sprague–Dawley rats | [80] |
| Biomimetic collagen, laminin, HA, and CS–proteoglycan biocomposites | Biomimetic hydrogels of collagen, laminin, HA, and CS-PGs developed to reproduce native ECM structure for the promotion of cell survival, neural differentiation, and neurite outgrowth. | [73] |
| Electrospun HA–polycaprolactone nanofiber bioscaffolds | Electrospun high-porosity nanofibrous scaffolds suitable for the growth of SH-SY5Y human neuroblastoma cells. | [84] |
| HA-poly-D-lysine hydrogel | Copolymer hydrogel with an open porous structure and viscoelastic properties similar to those of native brain tissue. Proposed as a promising scaffold for the repair of brain defects. | [78] |
| HA–laminin hydrogels | HA–laminin hydrogels implanted into brain defects promoted neurite extension and inhibited glial scar formation. | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melrose, J. Fractone Stem Cell Niche Components Provide Intuitive Clues in the Design of New Therapeutic Procedures/Biomatrices for Neural Repair. Int. J. Mol. Sci. 2022, 23, 5148. https://doi.org/10.3390/ijms23095148
Melrose J. Fractone Stem Cell Niche Components Provide Intuitive Clues in the Design of New Therapeutic Procedures/Biomatrices for Neural Repair. International Journal of Molecular Sciences. 2022; 23(9):5148. https://doi.org/10.3390/ijms23095148
Chicago/Turabian StyleMelrose, James. 2022. "Fractone Stem Cell Niche Components Provide Intuitive Clues in the Design of New Therapeutic Procedures/Biomatrices for Neural Repair" International Journal of Molecular Sciences 23, no. 9: 5148. https://doi.org/10.3390/ijms23095148