Is Infantile Hemangioma a Neuroendocrine Tumor?
Abstract
:1. Introduction
2. Results
2.1. Propranolol Treatment Reduces NA Level in IH
2.2. Catecholamine Biosynthetic Enzymes Are Expressed by IH Cells and Their Expression Levels Are Reduced by Propranolol
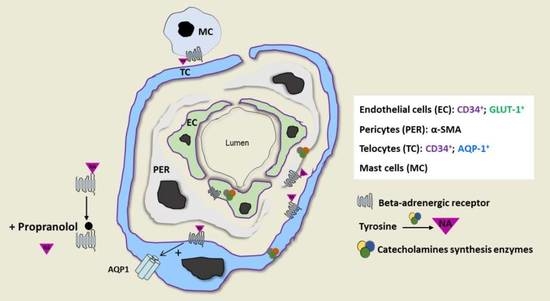
2.3. IH Sensitivity to Propranolol Depends on Crosstalk between IH-EC, PER and TC and the Presence of a Threshold Level of NA
2.4. Proliferative IH Have the Competency of Neuroendocrine Secretion
3. Discussion
4. Materials and Methods
4.1. Infantile Hemangioma and Foreskin Tissues
4.2. Catecholamines ELISA Assay
4.3. Immunohistochemistry
4.4. Protein Extraction and Western Blotting
4.5. IH Cell Sorting and Culture
4.6. Immunocytofluorescence
4.7. Electron Microscopy
4.8. In Vitro Matrigel Tube Formation Assay
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dasgupta, R.; Fishman, S.J. ISSVA classification. Semin. Pediatr. Surg. 2014, 23, 158–161. [Google Scholar] [CrossRef]
- Léauté-Labrèze, C.; Harper, J.I.; Hoeger, P.H. Infantile haemangioma. Lancet Lond. Engl. 2017, 390, 85–94. [Google Scholar] [CrossRef]
- Haggstrom, A.N.; Drolet, B.A.; Baselga, E.; Chamlin, S.L.; Garzon, M.C.; Horii, K.A.; Lucky, A.W.; Mancini, A.J.; Metry, D.W.; Newell, B.; et al. Prospective study of infantile hemangiomas: Clinical characteristics predicting complications and treatment. Pediatrics 2006, 118, 882–887. [Google Scholar] [CrossRef]
- Léauté-Labrèze, C.; de la Roque, E.D.; Hubiche, T.; Boralevi, F.; Thambo, J.-B.; Taïeb, A. Propranolol for Severe Hemangiomas of Infancy. N. Engl. J. Med. 2008, 358, 2649–2651. [Google Scholar] [CrossRef]
- Léauté-Labrèze, C.; Hoeger, P.; Mazereeuw-Hautier, J.; Guibaud, L.; Baselga, E.; Posiunas, G.; Phillips, R.J.; Caceres, H.; Lopez Gutierrez, J.C.; Ballona, R.; et al. A Randomized, Controlled Trial of Oral Propranolol in Infantile Hemangioma. N. Engl. J. Med. 2015, 372, 735–746. [Google Scholar] [CrossRef] [Green Version]
- Prey, S.; Voisard, J.-J.; Delarue, A.; Lebbe, G.; Taïeb, A.; Leaute-Labreze, C.; Ezzedine, K. Safety of Propranolol Therapy for Severe Infantile Hemangioma. JAMA 2016, 315, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, M.T.; Perkins, J.A.; Messner, A.H.; Chang, K.W. Propranolol for the treatment of airway hemangiomas: A case series and treatment algorithm. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 1043–1048. [Google Scholar] [CrossRef]
- Greenberger, S.; Bischoff, J. Infantile hemangioma-mechanism(s) of drug action on a vascular tumor. Cold Spring Harb. Perspect. Med. 2011, 1, a006460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storch, C.H.; Hoeger, P.H. Propranolol for infantile haemangiomas: Insights into the molecular mechanisms of action. Br. J. Dermatol. 2010, 163, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Chakroborty, D.; Sarkar, C.; Basu, B.; Dasgupta, P.S.; Basu, S. Catecholamines regulate tumor angiogenesis. Cancer Res. 2009, 69, 3727–3730. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.-K.; Li, P.; Guo, Z.-T.; Huang, Q.; Gao, Y. Propranolol induces regression of hemangioma cells via the down-regulation of the PI3K/Akt/eNOS/VEGF pathway. Pediatr. Blood Cancer 2015, 62, 1414–1420. [Google Scholar] [CrossRef]
- Shi, M.; Liu, D.; Yang, Z.; Guo, N. Central and peripheral nervous systems: Master controllers in cancer metastasis. Cancer Metastasis Rev. 2013, 32, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kang, J.H.; Jeong, K.J.; Lee, J.; Han, J.W.; Choi, W.S.; Kim, Y.K.; Kang, J.; Park, C.G.; Lee, H.Y. Norepinephrine induces VEGF expression and angiogenesis by a hypoxia-inducible factor-1α protein-dependent mechanism. Int. J. Cancer 2011, 128, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, D.; Yang, Z.; Sun, L.; Deng, Q.; Yang, S.; Qian, L.; Guo, L.; Yu, M.; Hu, M.; et al. Adrenergic signaling promotes angiogenesis through endothelial cell-tumor cell crosstalk. Endocr. Relat. Cancer 2014, 21, 783–795. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, C.; Chakroborty, D.; Basu, S. Neurotransmitters as regulators of tumor angiogenesis and immunity: The role of catecholamines. J. Neuroimmune Pharmacol. 2013, 8, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Sorriento, D.; Santulli, G.; Del Giudice, C.; Anastasio, A.; Trimarco, B.; Iaccarino, G. Endothelial cells are able to synthesize and release catecholamines both in vitro and in vivo. Hypertension 2012, 60, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prey, S.; Leaute-Labreze, C.; Pain, C.; Moisan, F.; Vergnes, P.; Loot, M.; Taieb, A.; Cario-Andre, M. Mast cells as possible targets of propranolol therapy: An immunohistological study of beta-adrenergic receptors in infantile haemangiomas. Histopathology 2014, 65, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, G.; Cipolletta, E.; Fiorillo, A.; Annecchiarico, M.; Ciccarelli, M.; Cimini, V.; Koch, W.J.; Trimarco, B. Beta(2)-adrenergic receptor gene delivery to the endothelium corrects impaired adrenergic vasorelaxation in hypertension. Circulation 2002, 106, 349–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dosanjh, A.; Chang, J.; Bresnick, S.; Zhou, L.; Reinisch, J.; Longaker, M.; Karasek, M. In vitro characteristics of neonatal hemangioma endothelial cells: Similarities and differences between normal neonatal and fetal endothelial cells. J. Cutan. Pathol. 2000, 27, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Boye, E.; Yu, Y.; Paranya, G.; Mulliken, J.B.; Olsen, B.R.; Bischoff, J. Clonality and altered behavior of endothelial cells from hemangiomas. J. Clin. Investig. 2001, 107, 745–752. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Varughese, J.; Brown, L.F.; Mulliken, J.B.; Bischoff, J. Increased Tie2 expression, enhanced response to angiopoietin-1, and dysregulated angiopoietin-2 expression in hemangioma-derived endothelial cells. Am. J. Pathol. 2001, 159, 2271–2280. [Google Scholar] [CrossRef] [Green Version]
- Dadras, S.S.; North, P.E.; Bertoncini, J.; Mihm, M.C.; Detmar, M. Infantile hemangiomas are arrested in an early developmental vascular differentiation state. Mod. Pathol. 2004, 17, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, Y.; Bischoff, J.; Mulliken, J.B.; Olsen, B.R. Differential expression of CD146 in tissues and endothelial cells derived from infantile haemangioma and normal human skin. J. Pathol. 2003, 201, 296–302. [Google Scholar] [CrossRef]
- Boscolo, E.; Bischoff, J. Vasculogenesis in infantile hemangioma. Angiogenesis 2009, 12, 197–207. [Google Scholar] [CrossRef]
- Chisholm, K.M.; Chang, K.W.; Truong, M.T.; Kwok, S.; West, R.B.; Heerema-McKenney, A.E. β-Adrenergic receptor expression in vascular tumors. Mod. Pathol. 2012, 25, 1446–1451. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; Sáez, F.J.; Díaz-Flores, L.; Valladares, F.; Madrid, J.F. CD34+ stromal cells/fibroblasts/fibrocytes/telocytes as a tissue reserve and a principal source of mesenchymal cells. Location, morphology, function and role in pathology. Histol. Histopathol. 2014, 29, 831–870. [Google Scholar] [CrossRef] [PubMed]
- Moisan, F.; Oucherif, S.; Kaulanjan-Checkmodine, P.; Prey, S.; Rousseau, B.; Bonneu, M.; Claverol, S.; Gontier, E.; Lacomme, S.; Dousset, L.; et al. Critical role of Aquaporin-1 and telocytes in infantile hemangioma response to propranolol beta blockade. Proc. Natl. Acad. Sci. USA 2021, 118, e2018690118. [Google Scholar] [CrossRef]
- North, P.E.; Waner, M.; Mizeracki, A.; Mihm, M.C., Jr. GLUT1: A newly discovered immunohistochemical marker for juvenile hemangiomas. Hum. Pathol. 2000, 31, 11–22. [Google Scholar] [CrossRef]
- Cohn, D.V.; Zangerle, R.; Fischer-Colbrie, R.; Chu, L.L.; Elting, J.J.; Hamilton, J.W.; Winkler, H. Similarity of secretory protein I from parathyroid gland to chromogranin A from adrenal medulla. Proc. Natl. Acad. Sci. USA 1982, 79, 6056–6059. [Google Scholar] [CrossRef] [Green Version]
- Huttner, W.B.; Gerdes, H.H.; Rosa, P. The granin-(chromogranin/secretogranin) family. Trends Biochem. Sci. 1991, 16, 27–30. [Google Scholar] [CrossRef]
- Borges, R.; Díaz-Vera, J.; Domínguez, N.; Arnau, M.R.; Machado, J.D. Chromogranins as regulators of exocytosis: Cgs as regulators of exocytosis. J. Neurochem. 2010, 114, 335–343. [Google Scholar] [CrossRef]
- Laguerre, F.; Anouar, Y.; Montero-Hadjadje, M. Chromogranin A in the early steps of the neurosecretory pathway. IUBMB Life 2020, 72, 524–532. [Google Scholar] [CrossRef]
- Jacob, R.; Gülch, R.; Kissling, G. Cardiac Adaptation to Hemodynamic Overload, Training and Stress; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 978-3-642-85326-5. [Google Scholar]
- Tuross, N.; Patrick, R.L. Effects of propranolol on catecholamine synthesis and uptake in the central nervous system of the rat. J. Pharmacol. Exp. Ther. 1986, 237, 739–745. [Google Scholar] [PubMed]
- Srivastava, M.; Kapoor, N.K. The effect of propranolol on rat brain catecholamine biosynthesis. J. Biosci. 1983, 5, 261–266. [Google Scholar] [CrossRef]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Madden, K.S.; Szpunar, M.J.; Brown, E.B. β-Adrenergic receptors (β-AR) regulate VEGF and IL-6 production by divergent pathways in high β-AR-expressing breast cancer cell lines. Breast Cancer Res. Treat. 2011, 130, 747–758. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Chen, S.; Xu, C.; Li, L.; Xiang, B. The use of propranolol in the treatment of infantile haemangiomas: An update on potential mechanisms of action. Br. J. Dermatol. 2015, 172, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, G.; Ciccarelli, M.; Sorriento, D.; Galasso, G.; Campanile, A.; Santulli, G.; Cipolletta, E.; Cerullo, V.; Cimini, V.; Altobelli, G.G.; et al. Ischemic Neoangiogenesis Enhanced by β2-Adrenergic Receptor Overexpression: A Novel Role for the Endothelial Adrenergic System. Circ. Res. 2005, 97, 1182–1189. [Google Scholar] [CrossRef] [Green Version]
- Ciccarelli, M.; Sorriento, D.; Cipolletta, E.; Santulli, G.; Fusco, A.; Zhou, R.-H.; Eckhart, A.D.; Peppel, K.; Koch, W.J.; Trimarco, B.; et al. Impaired neoangiogenesis in β2-adrenoceptor gene-deficient mice: Restoration by intravascular human β2-adrenoceptor gene transfer and role of NFκB and CREB transcription factors: β2AR and neoangiogenesis. Br. J. Pharmacol. 2011, 162, 712–721. [Google Scholar] [CrossRef] [Green Version]
- Taran, K.; Wnęk, A.; Kobos, J.; Andrzejewska, E.; Przewratil, P. Tissue and serum mRNA profile of MMPs-2/9 as a potential novel biomarker for the most individual approach in infantile hemangiomas and cancer disease. Immunobiology 2017, 222, 1035–1042. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, W.; Li, Y.-P.; Ren, J.-G.; Xu, N.; Liu, H.; Wang, F.-Q.; Sun, Z.-J.; Jia, J.; Zhao, Y.-F. Hypoxia-induced autophagy in endothelial cells: A double-edged sword in the progression of infantile haemangioma? Cardiovasc. Res. 2013, 98, 437–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaulanjan-Checkmodine, P.; Oucherif, S.; Prey, S.; Gontier, E.; Lacomme, S.; Loot, M.; Miljkovic-Licina, M.; Cario, M.; Léauté-Labrèze, C.; Taieb, A.; et al. Is Infantile Hemangioma a Neuroendocrine Tumor? Int. J. Mol. Sci. 2022, 23, 5140. https://doi.org/10.3390/ijms23095140
Kaulanjan-Checkmodine P, Oucherif S, Prey S, Gontier E, Lacomme S, Loot M, Miljkovic-Licina M, Cario M, Léauté-Labrèze C, Taieb A, et al. Is Infantile Hemangioma a Neuroendocrine Tumor? International Journal of Molecular Sciences. 2022; 23(9):5140. https://doi.org/10.3390/ijms23095140
Chicago/Turabian StyleKaulanjan-Checkmodine, Priscilla, Sandra Oucherif, Sorilla Prey, Etienne Gontier, Sabrina Lacomme, Maya Loot, Marijana Miljkovic-Licina, Muriel Cario, Christine Léauté-Labrèze, Alain Taieb, and et al. 2022. "Is Infantile Hemangioma a Neuroendocrine Tumor?" International Journal of Molecular Sciences 23, no. 9: 5140. https://doi.org/10.3390/ijms23095140