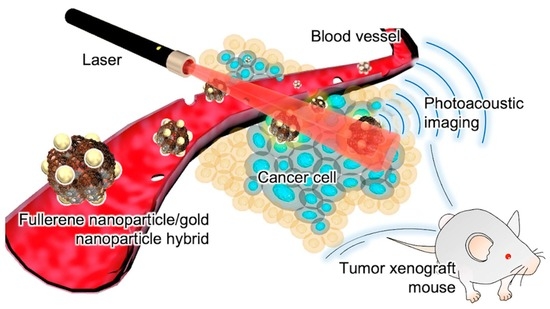
Theranostic Agent Combining Fullerene Nanocrystals and Gold Nanoparticles for Photoacoustic Imaging and Photothermal Therapy
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the C60@γ-CD Complex
4.3. Preparation of Fullerene Nanocrystal (FNCs)
4.4. Preparation of Fullerene Nanocrystal/Gold Nanoparticle Hybrid (FGNPs)
4.5. Wide Angle X-ray Scattering Measurement
4.6. Photothermal Heating Character
4.7. Photoacoustic Signal of Dispersion
4.8. Cell Viability Assay
4.9. Photothermal Therapy In Vitro
4.10. Cellular Uptake and Photoacoustic Imaging In Vitro
4.11. Biodistribution of FGNPs
4.12. Photoacoustic Imaging In Vivo
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, E.-K.; Kim, T.; Pail, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for Theranostcs: Recent Advances and Future Challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Shin, W.S.; Sunwoo, K.; Kim, W.Y.; Koo, S.; Bhuniya, S.; Kim, J.S. Small conjugate-based theranostic agents: An encouraging approach for cancer therapy. Chem. Soc. Rev. 2015, 44, 6670–6683. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.B.; Decuzzi, P. Harnessing Endogenous Stimuli for Responsive Materials in Theranostics. ACS Nano 2021, 15, 2068–2098. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Fu, Q.; Zhu, R.; Song, J.; Yang, H.; Chen, X. Photoacoustic Imaging: Contrast Agents and Their Biomedical Applications. Adv. Mater. 2019, 31, e1805875. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.; Beard, P.C.; Bohndiek, S.E. Contrast Agets for Molecular Photoacoustic Imaging. Nat. Methods 2016, 13, 639–650. [Google Scholar] [CrossRef] [Green Version]
- Mallidi, S.; Larson, T.; Tam, J.; Joshi, P.P.; Karpiouk, A.; Sokolov, K.; Emlianov, S. Multiwavelength Photoacoustic Imaging ad Plasmon Resonance Coupling of Gold Nanoparticles for Selective Detection of Cancer. Nano Lett. 2009, 9, 2825–2831. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Sun, R.; Yin, L.; Chai, Z.; Shi, H.; Gao, M. Light-Triggered Assembly of Gold Nanoparticles for Photothermal Therapy and Photoacoustic Imaging of Tumors In Vivo. Adv. Mater. 2017, 29, 1604894. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, L.; Sun, T.; Zhang, Y.; Liu, Y.; Gong, M.; Xu, Z.; Du, M.; Liu, Y.; Liu, G.; et al. Activatable NIR-II Plasmonic Nanotheranostics for Efficient Photoacoustic Imaging and Photothermal Cancer Therapy. Adv. Mater. 2021, 33, e2006532. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Z.; Lin, L.; Liu, F.; Wang, Y.; Guo, Z.; Li, Y.; Tian, H.; Chen, X. Porphyrin-based covalent organic framework nanoparticles for photoacoustic imaging-guided photodynamic and photothermal combination cancer therapy. Biomaterials 2019, 223, 119459. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Park, E.; Kang, Y.; Kwon, N.; Yang, M.; Lee, S.; Kim, W.J.; Kim, C.; Yoon, J. Supramolecular Phthalocyanine Assemblies for Improved Photoacoustic Imaging and Photothermal Therapy. Angew. Chem. 2020, 132, 8708–8712. [Google Scholar] [CrossRef]
- Wood, C.A.; Han, S.; Kim, C.S.; Wen, Y.; Sampaio, D.R.T.; Harris, J.T.; Homan, K.A.; Swain, J.L.; Emelianov, S.Y.; Sood, A.K.; et al. Clinically translatable quantitative molecular photoacoustic imaging with liposome-encapsulated ICG J-aggregates. Nat. Commun. 2021, 12, 5410. [Google Scholar] [CrossRef]
- De La Zerda, A.; Zavaleta, C.; Keren, S.; Vaithilingam, S.; Bodapati, S.; Liu, Z.; Levi, J.; Smith, B.; Ma, T.-J.; Oralkan, O.; et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat. Nanotechnol. 2008, 3, 557–562. [Google Scholar] [CrossRef]
- Krishna, V.; Singh, A.; Sharma, P.; Iwakuma, N.; Wang, Q.; Zhang, Q.; Knapik, J.; Jiang, H.; Grobmyer, S.R.; Koopman, B.; et al. Polyhydroxy Fullerenes for Non-Invasive Cancer Imaging and Therapy. Small 2010, 6, 2236–2241. [Google Scholar] [CrossRef]
- Shi, H.; Gu, R.; Xu, W.; Huang, H.; Xue, L.; Wang, W.; Zhang, Y.-W.; Si, W.; Dong, X. Near-Infrared Light-Harvesting Fullerene-Based Nanoparticles for Promoted Synergetic Tumor Phototheranostics. ACS Appl. Mater. Interfaces 2019, 11, 44970–44977. [Google Scholar] [CrossRef]
- Malavika, J.P.; Shobana, C.; Sunbagamoorthy, S.; Ganeshbabu, M.; Kumar, P.; Selvan, R.K. Green Synthesis of Multifunctional Carbon Quantum Dots: An Approach in Cancer Theranostics. Biomater. Adv. 2022, 212756. [Google Scholar] [CrossRef]
- Moon, H.; Kumar, D.; Kim, H.; Sim, C.; Chang, J.-H.; Kim, J.-M.; Kim, H.; Lim, D.-K. Amplified Photoacoustic Performance and Enhanced Photothermal Stability of Reduced Graphene Oxide Coated Gold Nanorods for Sensitive Photoacoustic Imaging. ACS Nano 2015, 9, 2711–2719. [Google Scholar] [CrossRef]
- Ikeda, A.; Iizuka, T.; Maekubo, N.; Nobusawa, K.; Sugikawa, K.; Koumoto, K.; Suzuki, T.; Nagasaki, T.; Akiyama, M. Water Solubilization of Fullerene Derivatives by β-(1,3-1,6)-D-Glucan and Their Photodynamic Activities toward Macrophages. Chem. Asian J. 2017, 12, 1069–1074. [Google Scholar] [CrossRef]
- Kawasaki, R.; Antoku, D.; Ohdake, R.; Sugikawa, K.; Ikeda, A. Bacterial elimination via the photodynamic activity of a fullerene/light-harvesting antenna molecule assembled system integrated into liposome membranes. Nanoscale Adv. 2020, 2, 4395–4399. [Google Scholar] [CrossRef]
- Yumoto, T.; Satake, S.; Hino, S.; Sugikawa, K.; Kawasaki, R.; Ikeda, A. Improved water solubility and photodynamic activity of hydroxy-modified porphyrins by complexation with cyclodextrin. Org. Biomol. Chem. 2020, 18, 6702–6709. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, R.; Yamana, K.; Shimada, R.; Sugikawa, K.; Ikeda, A. Water Solubilization and Thermal Stimuli-Triggered Release of Porphyrin Derivatives Using Thermoresponsive Polysaccharide Hydroxypropyl Cellulose for Mitochondria-Targeted Photodynamic Therapy. ACS Omega 2021, 6, 3209–3217. [Google Scholar] [CrossRef] [PubMed]
- Yamana, K.; Kawasaki, R.; Sugikawa, K.; Ikeda, A. Solubilization of Tetrahydroxyphenylchlorin in Water and Improved Photodynamic Activity after Complexation with Cyclic Oligo- and Polysaccharides. ACS Appl. Bio Mater. 2020, 3, 3217–3225. [Google Scholar] [CrossRef]
- Goto, Y.; Hino, S.; Sugikawa, K.; Kawasaki, R.; Ikeda, A. Water Solubilization of Phthalocyanine Derivatives via Interactions of Long Alkyl Chains and Cyclodextrins: Potential Complexes for Photodynamic Therapy. Asian J. Org. Chem. 2020, 9, 1589–1596. [Google Scholar] [CrossRef]
- Sugikawa, K.; Kozawa, K.; Ueda, M.; Ikeda, A. Stepwise Growth of Fullerene Nanoparticles through Guest Exchange of γ-Cyclodextrin Complexes in Water. Chem. A Eur. J. 2017, 23, 13704–13710. [Google Scholar] [CrossRef]
- Sugikawa, K.; Masuda, K.; Kozawa, K.; Kawasaki, R.; Ikeda, A. Fullerene–porphyrin hybrid nanoparticles that generate activated oxygen by photoirradiation. RSC Adv. 2021, 11, 1564–1568. [Google Scholar] [CrossRef]
- Miki, K.; Imaizumi, N.; Nogita, K.; Oe, M.; Mu, H.; Huo, W.; Harada, H.; Ohe, K. MMP-2-Activatable Photoacoustic Tumor Imaging Probes Based on Al- and Si-Naphthalocyanines. Bioconjugate Chem. 2021, 32, 1773–1781. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why Gold Nanoparticles Are More Precious Than Pretty Gold: Noble Metal Surface Plasmon Resonance and Nonradiative Properties of Nanocrystals of Different Shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Sudeep, P.K.; Ipe, B.I.; Thomas, K.G.; George, M.V. Fullerene-functionalized gold nanoparticles. A self-assembled photoactive antenna-metal nanocore assembly. Nano Lett. 2002, 2, 29–35. [Google Scholar]
- Lu, F.; Xiao, S.; Li, Y.; Song, Y.; Liu, H.; Li, H.; Zhuang, J.; Liu, Y.; Gan, L.; Zhu, D. Fullerene-functionalized gold core–shell nanoparticles: Preparation and optical limiting properties. Inorg. Chem. Commun. 2004, 7, 960–962. [Google Scholar] [CrossRef]
- Amendola, V.; Mattei, G.; Cusan, C.; Prato, M.; Meneghetti, M. Fullerene non-linear excited state absorption induced by gold nanoparticles light harvesting. Synth. Met. 2005, 155, 283–286. [Google Scholar] [CrossRef]
- Sugikawa, K.; Kadota, T.; Yasuhara, K.; Ikeda, A. Anisotropic Self-Assembly of Citrate-Coated Gold Nanoparticles on Fluidic Liposomes. Angew. Chem. Int. Ed. 2016, 55, 4059–4063. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, M.; Wangoo, N.; Jain, D.V.S.; Sharma, R.K. Quantification of adsorbed and dangling citrate ions on gold nanoparticle surface using thermogravimetric analysis. Sci. Rep. 2020, 10, 8213. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Fu, S.; Zhang, A. Facile synthesis of water-soluble fullerene (C60) nanoparticles via mussel-inspired chemistry as efficient antioxidants. Nanomaterials 2019, 9, 1647. [Google Scholar] [CrossRef] [Green Version]




| Dhy/nm a | PDI a | ζ-Potential /mV b | [C60]/mM | [Au]/ppm c | |
|---|---|---|---|---|---|
| FNCs | 84 ± 5 | 0.06 | −23.1 ± 2.3 | 1.0 | - |
| GNPs | 13 ± 1 | 0.04 | −28.1 ± 3.8 | - | 300 |
| FGNPs | 98 ± 5 | 0.15 | −12.1 ± 4.2 | 1.0 | 313 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawasaki, R.; Kondo, K.; Miura, R.; Yamana, K.; Isozaki, H.; Shimada, R.; Kawamura, S.; Hirano, H.; Nishimura, T.; Tarutani, N.; et al. Theranostic Agent Combining Fullerene Nanocrystals and Gold Nanoparticles for Photoacoustic Imaging and Photothermal Therapy. Int. J. Mol. Sci. 2022, 23, 4686. https://doi.org/10.3390/ijms23094686
Kawasaki R, Kondo K, Miura R, Yamana K, Isozaki H, Shimada R, Kawamura S, Hirano H, Nishimura T, Tarutani N, et al. Theranostic Agent Combining Fullerene Nanocrystals and Gold Nanoparticles for Photoacoustic Imaging and Photothermal Therapy. International Journal of Molecular Sciences. 2022; 23(9):4686. https://doi.org/10.3390/ijms23094686
Chicago/Turabian StyleKawasaki, Riku, Kosuke Kondo, Risako Miura, Keita Yamana, Hinata Isozaki, Risako Shimada, Shogo Kawamura, Hidetoshi Hirano, Tomoki Nishimura, Naoki Tarutani, and et al. 2022. "Theranostic Agent Combining Fullerene Nanocrystals and Gold Nanoparticles for Photoacoustic Imaging and Photothermal Therapy" International Journal of Molecular Sciences 23, no. 9: 4686. https://doi.org/10.3390/ijms23094686