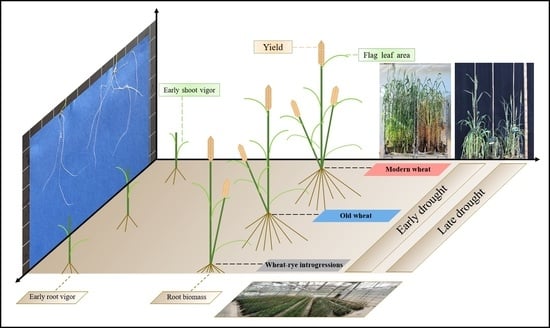
Climate Change Impact on Wheat Performance—Effects on Vigour, Plant Traits and Yield from Early and Late Drought Stress in Diverse Lines
Abstract
:1. Introduction
2. Results
2.1. Early Root and Shoot Development
2.2. Relationships between Drought Stresses and Plant Traits
2.3. Relationships among Investigated Traits
2.4. Genotypic Differences in Reactions to Drought Stresses
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Early Root and Shoot Phenotyping
4.3. Biotron Trial
4.4. Growing Conditions including Drought Stress
4.5. Morphological, Phenological and Yield Traits
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef] [Green Version]
- Cataldo, E.; Salvi, L.; Mattii, G.B. Effects of irrigation on ecophysiology, sugar content and thiol precursors (3-S-cysteinylhexan-1-ol and 3-S-glutathionylhexan-1-ol) on Vitis vinifera cv. Sauvignon Blanc. Plant Physiol. Biochem. 2021, 164, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Mattii, G.B. Effect of Agronomic Techniques on Aroma Composition of White Grapevines: A Review. Agronomy 2021, 11, 2027. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Plant Physiological Ecology; Springer Science & Business Media: New York, NY, USA, 2008. [Google Scholar]
- Fischer, R.; Maurer, R. Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Giunta, F.; Motzo, R.; Deidda, M. Effect of drought on yield and yield components of durum wheat and triticale in a Mediterranean environment. Field Crops Res. 1993, 33, 399–409. [Google Scholar] [CrossRef]
- Senapati, N.; Stratonovitch, P.; Paul, M.J.; Semenov, M.A. Drought tolerance during reproductive development is important for increasing wheat yield potential under climate change in Europe. J. Exp. Bot. 2019, 70, 2549–2560. [Google Scholar] [CrossRef] [Green Version]
- Van Ginkel, M.; Calhoun, D.; Gebeyehu, G.; Miranda, A.; Tian-You, C.; Lara, R.P.; Trethowan, R.; Sayre, K.; Crossa, J.; Rajaram, S. Plant traits related to yield of wheat in early, late, or continuous drought conditions. Euphytica 1998, 100, 109–121. [Google Scholar] [CrossRef]
- Chawade, A.; Armoniené, R.; Berg, G.; Brazauskas, G.; Frostgård, G.; Geleta, M.; Gorash, A.; Henriksson, T.; Himanen, K.; Ingver, A. A transnational and holistic breeding approach is needed for sustainable wheat production in the Baltic Sea region. Physiol. Plant. 2018, 164, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Gregorova, Z.; Kovacik, J.; Klejdus, B.; Maglovski, M.; Kuna, R.; Hauptvogel, P.; Matusikova, I. Drought-induced responses of physiology, metabolites, and PR proteins in Triticum aestivum. J. Agric. Food Chem. 2015, 63, 8125–8133. [Google Scholar] [CrossRef]
- Foulkes, M.; Sylvester-Bradley, R.; Weightman, R.; Snape, J. Identifying physiological traits associated with improved drought resistance in winter wheat. Field Crops Res. 2007, 103, 11–24. [Google Scholar] [CrossRef]
- Ehdaie, B.; Layne, A.P.; Waines, J.G. Root system plasticity to drought influences grain yield in bread wheat. Euphytica 2012, 186, 219–232. [Google Scholar] [CrossRef]
- Kumar, D.; Kushwaha, S.; Delvento, C.; Liatukas, Ž.; Vivekanand, V.; Svensson, J.T.; Henriksson, T.; Brazauskas, G.; Chawade, A. Affordable phenotyping of winter wheat under field and controlled conditions for drought tolerance. Agronomy 2020, 10, 882. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Qureshi, R.; Shaheen, H. Effects of pre-anthesis drought, heat and their combination on the growth, yield and physiology of diverse wheat (Triticum aestivum L.) genotypes varying in sensitivity to heat and drought stress. Sci. Rep. 2019, 9, 6955. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, G.C. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and other Food Crops in Temperature and Water Stress, Shanhua, Taiwan, 13–16 August 1992; pp. 257–270. [Google Scholar]
- White, J.W.; Conley, M.M. A flexible, low-cost cart for proximal sensing. Crop Sci. 2013, 53, 1646–1649. [Google Scholar] [CrossRef] [Green Version]
- Virlet, N.; Sabermanesh, K.; Sadeghi-Tehran, P.; Hawkesford, M.J. Field Scanalyzer: An automated robotic field phenotyping platform for detailed crop monitoring. Funct. Plant Biol. 2017, 44, 143–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutkoski, J.; Poland, J.; Mondal, S.; Autrique, E.; Pérez, L.G.; Crossa, J.; Reynolds, M.; Singh, R. Canopy temperature and vegetation indices from high-throughput phenotyping improve accuracy of pedigree and genomic selection for grain yield in wheat. G3 Genes Genomes Genet. 2016, 6, 2799–2808. [Google Scholar] [CrossRef] [Green Version]
- Leiva, F.; Vallenback, P.; Ekblad, T.; Johansson, E.; Chawade, A. Phenocave: An Automated, Standalone, and Affordable Phenotyping System for Controlled Growth Conditions. Plants 2021, 10, 1817. [Google Scholar] [CrossRef]
- Solomon, K.; Labuschagne, M. Morpho-physiological response of durum wheat genotypes to drought stress. S. Afr. J. Plant Soil 2009, 26, 141–146. [Google Scholar] [CrossRef] [Green Version]
- McFadden, E.; Sears, E. The origin of Triticum spelta and its free-thresching hexaploid relatives. J. Hered. 1964, 37, 107–116. [Google Scholar] [CrossRef]
- Dubcovsky, J.; Dvorak, J. Genome plasticity a key factor in the success of polyploid wheat under domestication. Science 2007, 316, 1862–1866. [Google Scholar] [CrossRef] [Green Version]
- Slafer, G.A.; Kernich, G.C. Have changes in yield (1900–1992) been accompanied by a decreased yield stability in Australian cereal production? Aust. J. Agric. Res. 1996, 47, 323–334. [Google Scholar] [CrossRef]
- Fufa, H.; Baenziger, P.S.; Beecher, B.; Graybosch, R.A.; Eskridge, K.M.; Nelson, L.A. Genetic improvement trends in agronomic performances and end-use quality characteristics among hard red winter wheat cultivars in Nebraska. Euphytica 2005, 144, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Molnar-Lang, M.; Ceoloni, C.; Dolezel, J. Alien introgression in wheat cytogenetics, molecular biology, and genomics. Cereal Res. Commun. 2016, 44, 535–536. [Google Scholar]
- Johansson, E.; Henriksson, T.; Prieto-Linde, M.L.; Andersson, S.; Ashraf, R.; Rahmatov, M. Diverse wheat-alien introgression lines as a basis for durable resistance and quality characteristics in bread wheat. Front. Plant Sci. 2020, 11, 1067. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Wellings, C.R.; Park, R.F. Wheat Rusts: An Atlas of Resistance Genes; CSIRO Publishing: Clayton, Australia, 1995. [Google Scholar]
- Friebe, B.; Jiang, J.; Raupp, W.; McIntosh, R.; Gill, B. Characterization of wheat-alien translocations conferring resistance to diseases and pests: Current status. Euphytica 1996, 91, 59–87. [Google Scholar] [CrossRef]
- Rahmatov, M.; Rouse, M.N.; Nirmala, J.; Danilova, T.; Friebe, B.; Steffenson, B.J.; Johansson, E. A new 2DS 2RL Robertsonian translocation transfers stem rust resistance gene Sr59 into wheat. Theor. Appl. Genet. 2016, 129, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Waines, J.; Ehdaie, B.; Sharma, S. Effect of origin of 1RS translocation on root biomass in wheats. In Proceedings of the Crop Science Society of America 49th Annual Meeting Program, Seattle, WA, USA, 1 November 2004. [Google Scholar]
- Sharma, S.; Bhat, P.R.; Ehdaie, B.; Close, T.J.; Lukaszewski, A.J.; Waines, J.G. Integrated genetic map and genetic analysis of a region associated with root traits on the short arm of rye chromosome 1 in bread wheat. Theor. Appl. Genet. 2009, 119, 783–793. [Google Scholar] [CrossRef] [Green Version]
- Palta, J.A.; Chen, X.; Milroy, S.P.; Rebetzke, G.J.; Dreccer, M.F.; Watt, M. Large root systems: Are they useful in adapting wheat to dry environments? Funct. Plant Biol. 2011, 38, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.C.; Nicolas, M.E. Early vigour: A yield-positive characteristic for wheat in drought-prone mediterranean-type environments. In Crop Improvement for Stress Tolerance; Behl, R.K., Singh, D.P., Lodhi, G.P., Eds.; CCSHAU, Hisar and MMB: New Delhi, India, 1998; pp. 47–62. [Google Scholar]
- Zhang, L.; Du, Y.-L.; Li, X.G. Modern wheat cultivars have greater root nitrogen uptake efficiency than old cultivars. J. Plant Nutr. Soil Sci. 2020, 183, 192–199. [Google Scholar] [CrossRef]
- Bektas, H.; Hohn, C.E.; Waines, J.G. Root and shoot traits of bread wheat (Triticum aestivum L.) landraces and cultivars. Euphytica 2016, 212, 297–311. [Google Scholar] [CrossRef]
- Bektas, H.; Waines, J.G. Root and shoot traits in parental, early and late generation Green Revolution wheats (Triticum spp.) under glasshouse conditions. Genet. Resour. Crop Evol. 2018, 65, 2003–2012. [Google Scholar] [CrossRef]
- Waines, J.G.; Ehdaie, B. Domestication and crop physiology: Roots of green-revolution wheat. Ann. Bot. 2007, 100, 991–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purnhauser, L.; Bóna, L.; Láng, L. Occurrence of 1BL. 1RS wheat-rye chromosome translocation and of Sr36/Pm6 resistance gene cluster in wheat cultivars registered in Hungary. Euphytica 2011, 179, 287–295. [Google Scholar] [CrossRef]
- Ren, T.-H.; Chen, F.; Yan, B.-J.; Zhang, H.-Q.; Ren, Z.-L. Genetic diversity of wheat–rye 1BL. 1RS translocation lines derived from different wheat and rye sources. Euphytica 2012, 183, 133–146. [Google Scholar] [CrossRef]
- Rahmatov, M.; Rouse, M.N.; Steffenson, B.J.; Andersson, S.C.; Wanyera, R.; Pretorius, Z.A.; Houben, A.; Kumarse, N.; Bhavani, S.; Johansson, E. Sources of stem rust resistance in wheat-alien introgression lines. Plant Dis. 2016, 100, 1101–1109. [Google Scholar] [CrossRef] [Green Version]
- Ren, T.; Ren, Z.; Yang, M.; Yan, B.; Tan, F.; Fu, S.; Tang, Z.; Li, Z. Novel source of 1RS from Baili rye conferred high resistance to diseases and enhanced yield traits to common wheat. Mol. Breed. 2018, 38, 101. [Google Scholar] [CrossRef]
- Ehdaie, B.; Whitkus, R.; Waines, J. Root biomass, water-use efficiency, and performance of wheat–rye translocations of chromosomes 1 and 2 in spring bread wheat ‘Pavon’. Crop Sci. 2003, 43, 710–717. [Google Scholar] [CrossRef]
- Sharma, A.; Sheikh, I.; Kumar, R.; Kumar, K.; Vyas, P.; Dhaliwal, H. Evaluation of end use quality and root traits in wheat cultivars associated with 1RS. 1BL translocation. Euphytica 2018, 214, 62. [Google Scholar] [CrossRef]
- Howell, T.; Moriconi, J.I.; Zhao, X.; Hegarty, J.; Fahima, T.; Santa-Maria, G.E.; Dubcovsky, J. A wheat/rye polymorphism affects seminal root length and yield across different irrigation regimes. J. Exp. Bot. 2019, 70, 4027–4037. [Google Scholar] [CrossRef]
- Liu, H.; Tang, H.; Ding, P.; Mu, Y.; Habib, A.; Liu, Y.; Jiang, Q.; Chen, G.; Kang, H.; Wei, Y. Effects of the 1BL/1RS translocation on 24 traits in a recombinant inbred line population. Cereal Res. Commun. 2020, 48, 225–232. [Google Scholar] [CrossRef]
- Kim, W.; Johnson, J.W.; Baenziger, P.S.; Lukaszewski, A.J.; Gaines, C.S. Agronomic Effect of Wheat-Rye Translocation Carrying Rye Chromatin (1R) From Different Sources. Crop Sci. 2004, 44, 1254–1258. [Google Scholar] [CrossRef]
- Villareal, R.L.; Bañuelos, O.; Mujeeb-Kazi, A.; Rajaram, S. Agronomic performance of chromosomes 1B and T1BL. 1RS near-isolines in the spring bread wheat Seri M82. Euphytica 1998, 103, 195–202. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Mares, D.; Marshall, D. Effect of 1B/1R chromosome translocation on melling and quality characteristics of bread wheats. Cereal Chem. 1987, 64, 72–76. [Google Scholar]
- Graybosch, R.; Peterson, C.; Hansen, L.; Worrall, D.; Shelton, D.; Lukaszewski, A. Comparative flour quality and protein characteristics of 1BL/1RS and 1AL/1RS wheat-rye translocation lines. J. Cereal Sci. 1993, 17, 95–106. [Google Scholar] [CrossRef]
- Fenn, D.; Lukow, O.; Bushuk, W.; Depauw, R. Milling and baking quality of 1BL/1RS translocation wheats. I: Effects of genotype and environment. Cereal Chem. 1994, 71, 189–195. [Google Scholar]
- Howell, T.; Hale, I.; Jankuloski, L.; Bonafede, M.; Gilbert, M.; Dubcovsky, J. Mapping a region within the 1RS. 1BL translocation in common wheat affecting grain yield and canopy water status. Theor. Appl. Genet. 2014, 127, 2695–2709. [Google Scholar] [CrossRef] [Green Version]
- Mathew, I.; Shimelis, H.; Mwadzingeni, L.; Zengeni, R.; Mutema, M.; Chaplot, V. Variance components and heritability of traits related to root: Shoot biomass allocation and drought tolerance in wheat. Euphytica 2018, 214, 1–12. [Google Scholar] [CrossRef]
- Atta, B.M.; Mahmood, T.; Trethowan, T.M. Relationship between root morphology and grain yield of wheat in north-western NSW, Australia. Aust. J. Crop Sci. 2013, 7, 2108–2115. [Google Scholar]
- Bai, C.; Ge, Y.; Ashton, R.; Evans, J.; Milne, A.; Hawkesford, M.; Whalley, W.; Parry, M.; Melichar, J.; Feuerhelm, D. The relationships between seedling root screens, root growth in the field and grain yield for wheat. Plant Soil 2019, 440, 311–326. [Google Scholar] [CrossRef]
- Wacker, L.; Jacomet, S.; Körner, C. Trends in biomass fractionation in wheat and barley from wild ancestors to modern cultivars. Plant Biol. 2002, 4, 258–265. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, L.; Xu, B.-C.; Li, F.-M. The relationship between competitive ability and yield stability in an old and a modern winter wheat cultivar. Plant Soil. 2011, 347, 7–23. [Google Scholar] [CrossRef]
- Wojciechowski, T.; Gooding, M.; Ramsay, L.; Gregory, P. The effects of dwarfing genes on seedling root growth of wheat. J. Exp. Bot. 2009, 60, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.H.; Rebetzke, G.J.; Chandler, P.; Bonnett, D.; Spielmeyer, W.; Richards, R.A. The effect of different height reducing genes on the early growth of wheat. Funct. Plant Biol. 2004, 31, 583–589. [Google Scholar] [CrossRef]
- Figueroa-Bustos, V.; Palta, J.A.; Chen, Y.; Siddique, K.H. Characterization of root and shoot traits in wheat cultivars with putative differences in root system size. Agronomy 2018, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Friedli, C.N.; Abiven, S.; Fossati, D.; Hund, A. Modern wheat semi-dwarfs root deep on demand: Response of rooting depth to drought in a set of Swiss era wheats covering 100 years of breeding. Euphytica 2019, 215, 85. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, R.; Farshadfar, E.; Aghaee-Sarbarzeh, M.; Sutka, J. Locating QTLs controlling drought tolerance criteria in rye using disomic addition lines. Cereal Res. Commun. 2003, 31, 257–264. [Google Scholar] [CrossRef]
- Aniol, A. Chromosomal location of aluminium tolerance genes in rye. Plant Breed 2004, 123, 132–136. [Google Scholar] [CrossRef]
- Gallego, F.; Lopez-Solanilla, E.; Figueiras, A.; Benito, C. Chromosomal location of PCR fragments as a source of DNA markers linked to aluminium tolerance genes in rye. Theor. Appl. Genet. 1998, 96, 426–434. [Google Scholar] [CrossRef]
- Ma, J.F.; Taketa, S.; Yang, Z.M. Aluminum tolerance genes on the short arm of chromosome 3R are linked to organic acid release in triticale. Plant Physiol. 2000, 122, 687–694. [Google Scholar] [CrossRef] [Green Version]
- Aniol, A.; Gustafson, J. Chromosome location of genes controlling aluminum tolerance in wheat, rye, and triticale. Can. J. Genet. Cytol. 1984, 26, 701–705. [Google Scholar] [CrossRef]
- Matos, M.; Camacho, M.; Pérez-Flores, V.; Pernaute, B.; Pinto-Carnide, O.; Benito, C. A new aluminum tolerance gene located on rye chromosome arm 7RS. Theor. Appl. Genet. 2005, 111, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B. Alteration of drought tolerance of winter wheat caused by translocation of rye chromosome segment 1RS. Cereal Res. Commun. 2008, 36, 269–278. [Google Scholar] [CrossRef]
- Barnabas, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, F.; Ahmad, R.; Waraich, E.; Naeem, M.; Shabbir, R. Nutrient uptake, physiological responses, and yield attributes of wheat (Triticum aestivum L.) exposed to early and late drought stress. J. Plant Nutr. 2012, 35, 961–974. [Google Scholar] [CrossRef]
- Schnyder, H.; Baum, U. Growth of the grain of wheat (Triticum aestivum L.). The relationship between water content and dry matter accumulation. Eur. J. Agron. 1992, 1, 51–57. [Google Scholar] [CrossRef]
- Gooding, M.; Ellis, R.; Shewry, P.; Schofield, J. Effects of restricted water availability and increased temperature on the grain filling, drying and quality of winter wheat. J. Cereal Sci. 2003, 37, 295–309. [Google Scholar] [CrossRef]
- Fábián, A.; Jäger, K.; Rakszegi, M.; Barnabás, B. Embryo and endosperm development in wheat (Triticum aestivum L.) kernels subjected to drought stress. Plant Cell Rep. 2011, 30, 551–563. [Google Scholar] [CrossRef]
- Egli, D.B. Seed Biology and Yield of Grain Crops; CABI: Wallingford, UK, 2017. [Google Scholar]
- Ellis, R.; Pieta Filho, C. The development of seed quality in spring and winter cultivars of barley and wheat. Seed Sci. Res. 1992, 2, 9–15. [Google Scholar] [CrossRef]
- Hassan, U.A.; Ogunlela, V.B.; Sinha, T.D. Agronomic Performance of Wheat (Triticum aestivum L.) as Influenced by Moisture Stress at Various Growth Stages and Seeding Rate. J. Agron. Crop Sci. 1987, 158, 172–180. [Google Scholar] [CrossRef]
- Rajaram, S.; Braun, H.-J.; van Ginkel, M. CIMMYT’s approach to breed for drought tolerance. In Adaptation in Plant Breeding; Springer: Berlin/Heidelberg, Germany, 1997; pp. 161–167. [Google Scholar]
- Bruckner, P.; Frohberg, R. Stress tolerance and adaptation in spring wheat 1. Crop Sci. 1987, 27, 31–36. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance–is it really a complex trait? Funct. Plant Biol. 2011, 38, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Sivamani, E.; Bahieldin, A.; Wraith, J.M.; Al-Niemi, T.; Dyer, W.E.; Ho, T.-H.D.; Qu, R. Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene. Plant Sci. 2000, 155, 1–9. [Google Scholar] [CrossRef]
- Ried, J.L.; Walker-Simmons, M.K. Group 3 Late Embryogenesis Abundant Proteins in Desiccation-Tolerant Seedlings of Wheat (Triticum aestivum L.). Plant Physiol. 1993, 102, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Chen, S.; Liu, G. Enhanced drought tolerance in transgenic Leymus chinensis plants with constitutively expressed wheat TaLEA3. Biotechnol. Lett. 2009, 31, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.S.I.; Li, X.; Li, H.-P.; Huang, T.; Gao, C.-S.; Guo, M.-W.; Cheng, W.; Zhao, G.-Y.; Liao, Y.-C. A rice stress-responsive NAC gene enhances tolerance of transgenic wheat to drought and salt stresses. Plant Science 2013, 203, 33–40. [Google Scholar] [CrossRef]
- Brini, F.; Hanin, M.; Mezghani, I.; Berkowitz, G.A.; Masmoudi, K. Overexpression of wheat Na+/H+ antiporter TNHX1 and H+-pyrophosphatase TVP1 improve salt- and drought-stress tolerance in Arabidopsis thaliana plants. J. Exp. Bot. 2007, 58, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Johansson, E.; Prieto-Linde, M.L.; Larsson, H. Locally Adapted and Organically Grown Landrace and Ancient Spring Cereals—A Unique Source of Minerals in the Human Diet. Foods 2021, 10, 393. [Google Scholar] [CrossRef]
- Merker, A. The rye genome in wheat breeding. Hereditas 1984, 100, 183–191. [Google Scholar] [CrossRef]
- Thomas, C.L.; Graham, N.; Hayden, R.; Meacham, M.C.; Neugebauer, K.; Nightingale, M.; Dupuy, L.X.; Hammond, J.P.; White, P.J.; Broadley, M.R. High-throughput phenotyping (HTP) identifies seedling root traits linked to variation in seed yield and nutrient capture in field-grown oilseed rape (Brassica napus L.). Ann. Bot. 2016, 118, 655–665. [Google Scholar] [CrossRef] [Green Version]
- Pound, M.P.; French, A.P.; Atkinson, J.A.; Wells, D.M.; Bennett, M.J.; Pridmore, T. RootNav: Navigating images of complex root architectures. Plant Physiol. 2013, 162, 1802–1814. [Google Scholar] [CrossRef] [Green Version]
- Armoniené, R.; Odilbekov, F.; Vivekanand, V.; Chawade, A. Affordable Imaging Lab for Noninvasive Analysis of Biomass and Early Vigour in Cereal Crops. BioMed Res. Int. 2018, 2018, 5713158. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Pantalião, G.F.; Narciso, M.; Guimarães, C.; Castro, A.; Colombari, J.M.; Breseghello, F.; Rodrigues, L.; Vianello, R.P.; Borba, T.O.; Brondani, C. Genome wide association study (GWAS) for grain yield in rice cultivated under water deficit. Genetica 2016, 144, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Team, R. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2015; 700p. [Google Scholar]






| EDS | LDS | ||||
|---|---|---|---|---|---|
| Genotype | STI | Genetic Background | Genotype | STI | Genetic Background |
| 257 | 1.29 | 3R | 238 | 0.67 | 2R |
| 256 | 1.25 | 3R | 281 | 0.66 | modern |
| 244 | 1.18 | 3R | 204 | 0.58 | old |
| 227 | 0.98 | 1RS | 273 | 0.57 | modern |
| 202 | 0.97 | old | 201 | 0.57 | old |
| 208 | 0.97 | old | 270 | 0.51 | 2R |
| 254 | 0.97 | 3R | 217 | 0.44 | 1R |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Y.; Chawade, A.; Kuktaite, R.; Johansson, E. Climate Change Impact on Wheat Performance—Effects on Vigour, Plant Traits and Yield from Early and Late Drought Stress in Diverse Lines. Int. J. Mol. Sci. 2022, 23, 3333. https://doi.org/10.3390/ijms23063333
Lan Y, Chawade A, Kuktaite R, Johansson E. Climate Change Impact on Wheat Performance—Effects on Vigour, Plant Traits and Yield from Early and Late Drought Stress in Diverse Lines. International Journal of Molecular Sciences. 2022; 23(6):3333. https://doi.org/10.3390/ijms23063333
Chicago/Turabian StyleLan, Yuzhou, Aakash Chawade, Ramune Kuktaite, and Eva Johansson. 2022. "Climate Change Impact on Wheat Performance—Effects on Vigour, Plant Traits and Yield from Early and Late Drought Stress in Diverse Lines" International Journal of Molecular Sciences 23, no. 6: 3333. https://doi.org/10.3390/ijms23063333